How To Calculate Titration Results . A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Can be used to calculate the. Our titration calculator will help you never have to ask how do i calculate titrations? again. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. , or the volume of solution needed. , or the volume of solution needed to. Can be used to calculate the. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. To determine the concentration of one of the solutions in mol/dm. Titration calculations are used to find the concentration of unknown solutions. They can also be used to calculate the ph after a given point during a titration. To determine the reacting volumes of solutions of an acid and alkali by titration. The amount of added titrant is determined from its concentration and volume: First determine the moles of \(\ce{naoh}\) in the reaction.
from www.youtube.com
, or the volume of solution needed to. To determine the reacting volumes of solutions of an acid and alkali by titration. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. Can be used to calculate the. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Our titration calculator will help you never have to ask how do i calculate titrations? again. First determine the moles of \(\ce{naoh}\) in the reaction. The amount of added titrant is determined from its concentration and volume: They can also be used to calculate the ph after a given point during a titration.
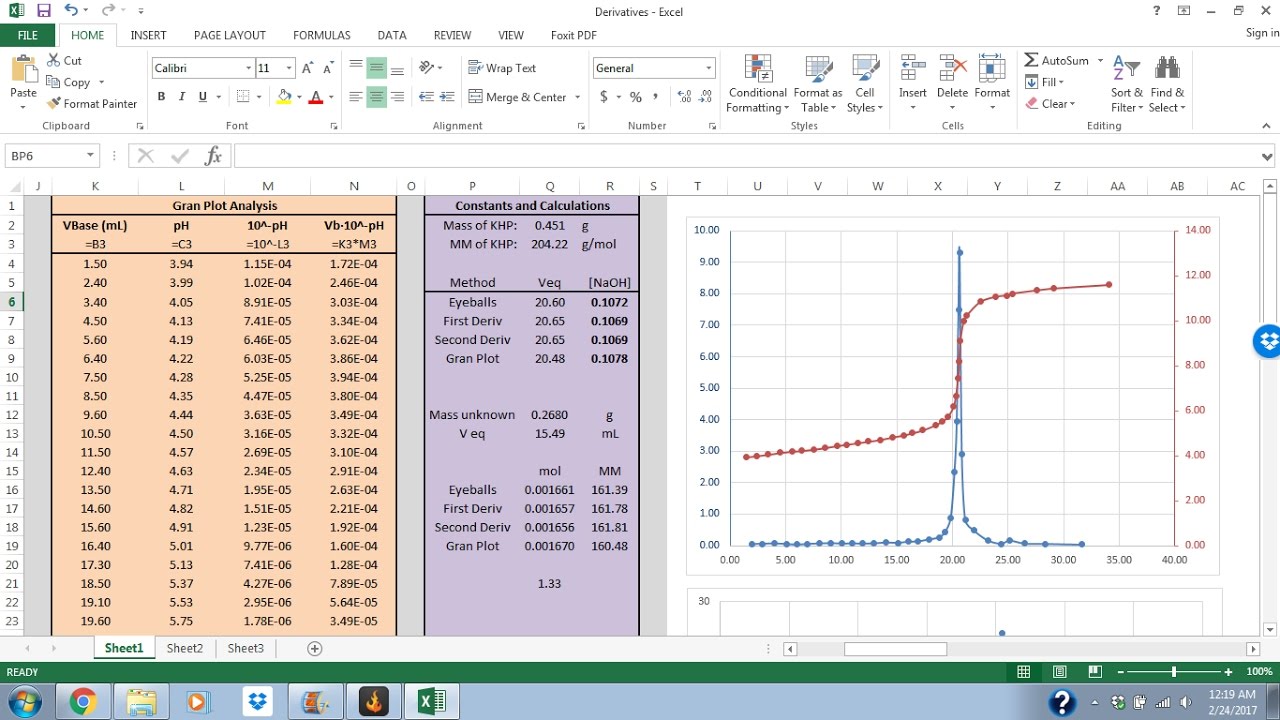
Excel Tutorial 2 Titration Analysis YouTube
How To Calculate Titration Results Our titration calculator will help you never have to ask how do i calculate titrations? again. Our titration calculator will help you never have to ask how do i calculate titrations? again. Can be used to calculate the. First determine the moles of \(\ce{naoh}\) in the reaction. The amount of added titrant is determined from its concentration and volume: N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. , or the volume of solution needed. To determine the reacting volumes of solutions of an acid and alkali by titration. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Titration calculations are used to find the concentration of unknown solutions. Can be used to calculate the. , or the volume of solution needed to. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. To determine the concentration of one of the solutions in mol/dm. They can also be used to calculate the ph after a given point during a titration.
From oneclass.com
OneClass For your fine titration record the following results How To Calculate Titration Results , or the volume of solution needed to. To determine the concentration of one of the solutions in mol/dm. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. They can also be used to calculate the ph after a given point during a titration. , or the volume of solution needed. To determine the reacting volumes of solutions. How To Calculate Titration Results.
From www.youtube.com
How to calculate uncertainty in titration YouTube How To Calculate Titration Results Our titration calculator will help you never have to ask how do i calculate titrations? again. First determine the moles of \(\ce{naoh}\) in the reaction. To determine the concentration of one of the solutions in mol/dm. The amount of added titrant is determined from its concentration and volume: Can be used to calculate the. Titration calculations are used to find. How To Calculate Titration Results.
From www.youtube.com
How to Calculate Concentration using Titration Example in Chemistry How To Calculate Titration Results To determine the concentration of one of the solutions in mol/dm. Our titration calculator will help you never have to ask how do i calculate titrations? again. Can be used to calculate the. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. To determine the reacting volumes of solutions of an acid and alkali by titration. They can. How To Calculate Titration Results.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation How To Calculate Titration Results First determine the moles of \(\ce{naoh}\) in the reaction. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. To determine the reacting volumes of solutions of an acid and alkali by titration. To determine the concentration of one of the solutions in mol/dm. Our titration calculator will help you. How To Calculate Titration Results.
From www.youtube.com
A Simple Guide to Titration Calculations Part 1 Concentration YouTube How To Calculate Titration Results A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The amount of added titrant is determined from its concentration and volume: N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. From the mole ratio, calculate the moles. How To Calculate Titration Results.
From www.youtube.com
Year 12 Chemistry Revision Titrating Using the Burette to make an How To Calculate Titration Results They can also be used to calculate the ph after a given point during a titration. Our titration calculator will help you never have to ask how do i calculate titrations? again. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Can be used to calculate the. A titration is a laboratory technique used to precisely measure molar. How To Calculate Titration Results.
From www.youtube.com
3.5 tabulating of results and calculating average titre YouTube How To Calculate Titration Results N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. Can be used to calculate the. To determine the reacting volumes of solutions of an acid and alkali by titration. Titration calculations are used to find the concentration of unknown solutions. Can be used to calculate the. They can. How To Calculate Titration Results.
From www.numerade.com
SOLVED To explain how the result of a titration is affected if you How To Calculate Titration Results The amount of added titrant is determined from its concentration and volume: From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. To determine the concentration of one of the solutions in mol/dm. , or the volume of solution needed to. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a. How To Calculate Titration Results.
From www.youtube.com
How to calculate titration results? l Titration Calculation l Chemistry How To Calculate Titration Results Can be used to calculate the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The amount of added titrant is determined from its concentration and volume: Titration calculations are used to find the concentration of unknown solutions. , or the volume of solution needed to. To determine the. How To Calculate Titration Results.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download How To Calculate Titration Results They can also be used to calculate the ph after a given point during a titration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. First determine the moles of \(\ce{naoh}\) in the reaction. , or the volume of solution needed to. Can be used to calculate the. Titration. How To Calculate Titration Results.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 How To Calculate Titration Results To determine the reacting volumes of solutions of an acid and alkali by titration. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. , or the volume of solution needed to. They can also be used to calculate the ph after a given point during a titration. Can be used to calculate the. Our titration calculator will help. How To Calculate Titration Results.
From www.xylemanalytics.com
How to get correct and reproducible results in titration How To Calculate Titration Results They can also be used to calculate the ph after a given point during a titration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. First determine the moles of \(\ce{naoh}\) in the reaction. , or the volume of solution needed to. From the mole ratio, calculate the moles. How To Calculate Titration Results.
From www.youtube.com
Titration calculation example Chemistry Khan Academy YouTube How To Calculate Titration Results Can be used to calculate the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. The amount of added titrant is determined from its concentration and volume: To. How To Calculate Titration Results.
From www.youtube.com
Titration Calculations YouTube How To Calculate Titration Results , or the volume of solution needed to. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. First determine the moles of \(\ce{naoh}\) in the reaction. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration calculations are used to find the concentration of unknown solutions. Our. How To Calculate Titration Results.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog How To Calculate Titration Results N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. The amount of added titrant is determined from its concentration and volume: To determine the reacting volumes of solutions of an acid and alkali by titration. To determine the concentration of one of the solutions in mol/dm. Our titration. How To Calculate Titration Results.
From childhealthpolicy.vumc.org
🐈 Titration experiment results. How do you report a titration How To Calculate Titration Results Titration calculations are used to find the concentration of unknown solutions. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. To determine the reacting volumes of solutions of an acid and alkali by titration. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. , or the volume. How To Calculate Titration Results.
From www.youtube.com
Titration Calculations AQA GCSE Chemistry YouTube How To Calculate Titration Results To determine the concentration of one of the solutions in mol/dm. First determine the moles of \(\ce{naoh}\) in the reaction. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Our titration calculator will help you never have. How To Calculate Titration Results.
From www.youtube.com
Excel Tutorial 2 Titration Analysis YouTube How To Calculate Titration Results Titration calculations are used to find the concentration of unknown solutions. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Our titration calculator will help you never have to ask how do i calculate titrations? again. , or the volume of solution needed to. To determine the reacting volumes. How To Calculate Titration Results.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download How To Calculate Titration Results , or the volume of solution needed. Can be used to calculate the. To determine the reacting volumes of solutions of an acid and alkali by titration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The amount of added titrant is determined from its concentration and volume: Titration. How To Calculate Titration Results.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 How To Calculate Titration Results Titration calculations are used to find the concentration of unknown solutions. To determine the concentration of one of the solutions in mol/dm. Can be used to calculate the. The amount of added titrant is determined from its concentration and volume: First determine the moles of \(\ce{naoh}\) in the reaction. Our titration calculator will help you never have to ask how. How To Calculate Titration Results.
From www.youtube.com
A short guide to titration calculations YouTube How To Calculate Titration Results Titration calculations are used to find the concentration of unknown solutions. , or the volume of solution needed. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Can be used to calculate the. Our titration calculator will help you never have to ask how do i calculate titrations? again.. How To Calculate Titration Results.
From www.showme.com
Titration calculation Science, Chemistry, Physical Chemistry ShowMe How To Calculate Titration Results To determine the concentration of one of the solutions in mol/dm. , or the volume of solution needed. The amount of added titrant is determined from its concentration and volume: N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. They can also be used to calculate the ph. How To Calculate Titration Results.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube How To Calculate Titration Results , or the volume of solution needed. Our titration calculator will help you never have to ask how do i calculate titrations? again. First determine the moles of \(\ce{naoh}\) in the reaction. , or the volume of solution needed to. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.. How To Calculate Titration Results.
From printablehaferbrotwp.z21.web.core.windows.net
How To Do Titrations In Chemistry How To Calculate Titration Results , or the volume of solution needed. The amount of added titrant is determined from its concentration and volume: , or the volume of solution needed to. To determine the concentration of one of the solutions in mol/dm. Our titration calculator will help you never have to ask how do i calculate titrations? again. Can be used to calculate the.. How To Calculate Titration Results.
From childhealthpolicy.vumc.org
🐈 Titration experiment results. How do you report a titration How To Calculate Titration Results Our titration calculator will help you never have to ask how do i calculate titrations? again. First determine the moles of \(\ce{naoh}\) in the reaction. Titration calculations are used to find the concentration of unknown solutions. Can be used to calculate the. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in. How To Calculate Titration Results.
From chem4three.blogspot.ca
CHEMISTRY 11 TITRATIONS How To Calculate Titration Results From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Titration calculations are used to find the concentration of unknown solutions. They can also be used to calculate the ph after a given point during a titration. Can be used to calculate the. Our titration calculator will help you never have to ask how do i calculate titrations? again.. How To Calculate Titration Results.
From www.youtube.com
Total Alkalinity Titration Method and Calculations YouTube How To Calculate Titration Results Can be used to calculate the. To determine the concentration of one of the solutions in mol/dm. The amount of added titrant is determined from its concentration and volume: Our titration calculator will help you never have to ask how do i calculate titrations? again. They can also be used to calculate the ph after a given point during a. How To Calculate Titration Results.
From noel-kmclean.blogspot.com
Calculate Molarity of Acid in Titration How To Calculate Titration Results They can also be used to calculate the ph after a given point during a titration. Can be used to calculate the. To determine the reacting volumes of solutions of an acid and alkali by titration. The amount of added titrant is determined from its concentration and volume: A titration is a laboratory technique used to precisely measure molar concentration. How To Calculate Titration Results.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations How To Calculate Titration Results They can also be used to calculate the ph after a given point during a titration. Titration calculations are used to find the concentration of unknown solutions. To determine the concentration of one of the solutions in mol/dm. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. Our. How To Calculate Titration Results.
From theedge.com.hk
Chemistry How To Titration The Edge How To Calculate Titration Results Titration calculations are used to find the concentration of unknown solutions. First determine the moles of \(\ce{naoh}\) in the reaction. Our titration calculator will help you never have to ask how do i calculate titrations? again. , or the volume of solution needed to. , or the volume of solution needed. N (mol) = c (mol /l) * v (l). How To Calculate Titration Results.
From mungfali.com
Acid Base Titration Calculation How To Calculate Titration Results A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. , or the volume of solution needed. To determine the concentration of one of the solutions in mol/dm. From. How To Calculate Titration Results.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube How To Calculate Titration Results They can also be used to calculate the ph after a given point during a titration. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Titration calculations are used to find the concentration of unknown solutions. To determine the concentration of one of the solutions in mol/dm. , or the volume of solution needed to. The amount of. How To Calculate Titration Results.
From www.youtube.com
An Introduction To Titration Calculations (GCSE Chemistry) YouTube How To Calculate Titration Results The amount of added titrant is determined from its concentration and volume: Titration calculations are used to find the concentration of unknown solutions. Can be used to calculate the. They can also be used to calculate the ph after a given point during a titration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown. How To Calculate Titration Results.
From www.sliderbase.com
Titration How To Calculate Titration Results A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration calculations are used to find the concentration of unknown solutions. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. They can also be used to calculate the. How To Calculate Titration Results.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube How To Calculate Titration Results Can be used to calculate the. To determine the concentration of one of the solutions in mol/dm. The amount of added titrant is determined from its concentration and volume: Our titration calculator will help you never have to ask how do i calculate titrations? again. To determine the reacting volumes of solutions of an acid and alkali by titration. Titration. How To Calculate Titration Results.