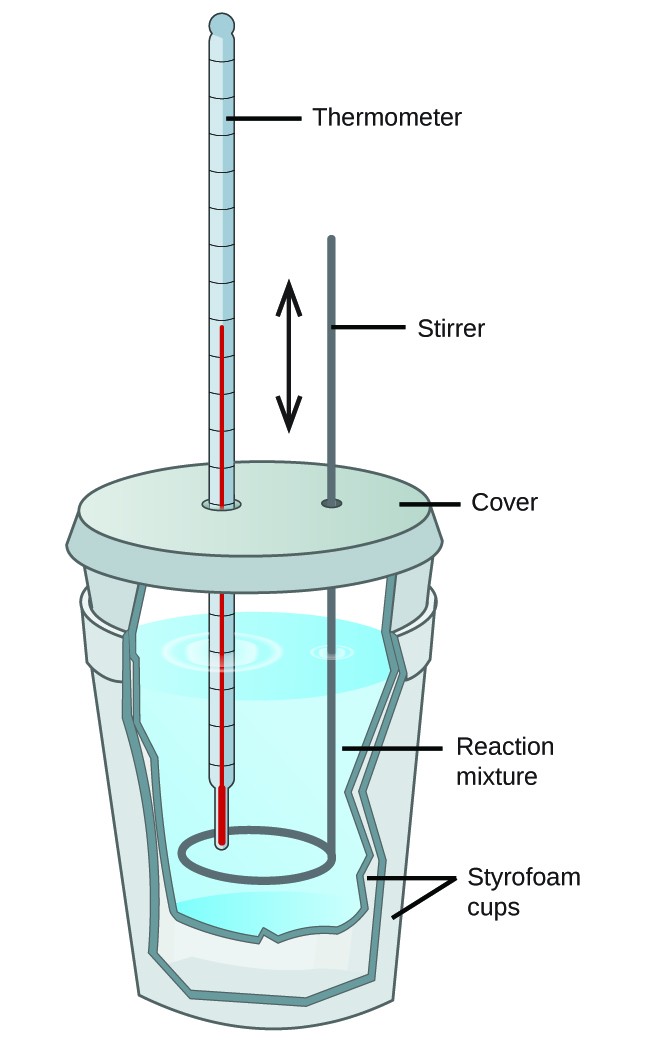
Explain About Calorimetry With Examples . The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer. In this article, we will. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate the molar heat of enthalpy for a. To do so, the heat is exchanged with a calibrated object. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and.
from courses.lumenlearning.com
Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. In this article, we will. To do so, the heat is exchanged with a calibrated object. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. Calculate the molar heat of enthalpy for a. Calorimetry is used to measure amounts of heat transferred to or from a substance.
Calorimetry Chemistry for Majors
Explain About Calorimetry With Examples One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. In this article, we will. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry is used to measure amounts of heat transferred to or from a substance. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer. Calculate the molar heat of enthalpy for a. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. To do so, the heat is exchanged with a calibrated object.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Explain About Calorimetry With Examples In this article, we will. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate. Explain About Calorimetry With Examples.
From dxojkdaez.blob.core.windows.net
Calorimetry Physics Examples at Jeremy Wetmore blog Explain About Calorimetry With Examples One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat. Explain About Calorimetry With Examples.
From www.slideserve.com
PPT Chem. 412 Phys. Chem. I PowerPoint Presentation, free download ID9205684 Explain About Calorimetry With Examples To do so, the heat is exchanged with a calibrated object. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. In this article, we will. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer. Calorimetry is used to. Explain About Calorimetry With Examples.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Explain About Calorimetry With Examples Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calculate the molar heat of enthalpy for a. Calorimetry is a branch of science concerned with measuring a body’s state. Explain About Calorimetry With Examples.
From www.youtube.com
AP Chemistry Thermochemical Equations and Calorimetry YouTube Explain About Calorimetry With Examples Calculate the molar heat of enthalpy for a. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Calorimetry is used to measure amounts of heat transferred to or from a substance. One technique we can use to measure the amount of heat involved in a chemical or physical. Explain About Calorimetry With Examples.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Explain About Calorimetry With Examples Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. In this article, we will. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. Explain About Calorimetry With Examples.
From www.youtube.com
050 Calorimetry YouTube Explain About Calorimetry With Examples Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. To do so, the heat is exchanged with a calibrated object. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Calculate the molar heat of enthalpy for a. Calorimetry. Explain About Calorimetry With Examples.
From klaxzllai.blob.core.windows.net
Calorimetry Equation Examples at Grace Smallwood blog Explain About Calorimetry With Examples Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer. In this article, we will. To. Explain About Calorimetry With Examples.
From www.slideshare.net
Lesson Enthalpy and Calorimetry Explain About Calorimetry With Examples Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Calculate the molar heat of enthalpy for. Explain About Calorimetry With Examples.
From www.slideserve.com
PPT Heat PowerPoint Presentation, free download ID1550332 Explain About Calorimetry With Examples Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. To do so, the heat is exchanged with a calibrated object. Calculate the molar heat of enthalpy for a. In this article, we will. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Explain About Calorimetry With Examples.
From www.studocu.com
Calorimetry Examples in Chemistry Chapter 9 Studocu Explain About Calorimetry With Examples Calculate the molar heat of enthalpy for a. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on. Explain About Calorimetry With Examples.
From www.studypool.com
SOLUTION Chemistry cit u calorimetry definition examples sample problems and etc Studypool Explain About Calorimetry With Examples Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its. Explain About Calorimetry With Examples.
From www.youtube.com
Physics 9.09b Calorimetry Example 1 YouTube Explain About Calorimetry With Examples Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. To do so, the heat is exchanged with a calibrated object. In this article, we will. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. Calorimetry measures enthalpy changes. Explain About Calorimetry With Examples.
From studylib.net
Calorimetry Explain About Calorimetry With Examples Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. In this article, we will. One technique we can use to measure the amount of heat involved in a chemical. Explain About Calorimetry With Examples.
From www.scribd.com
calorimetry Explain About Calorimetry With Examples To do so, the heat is exchanged with a calibrated object. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. Calculate the molar heat of enthalpy for a. Calorimeters. Explain About Calorimetry With Examples.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Explain About Calorimetry With Examples Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. To do so, the heat is exchanged with a calibrated object. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Calorimetry measures enthalpy changes during chemical processes, where the magnitude. Explain About Calorimetry With Examples.
From joiddcrwa.blob.core.windows.net
What Is Calorimetry Example at Donovan blog Explain About Calorimetry With Examples Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature. Explain About Calorimetry With Examples.
From www.slideserve.com
PPT Calorimetry C ontents Basic Concept Example Whiteboards Bomb Calorimeters PowerPoint Explain About Calorimetry With Examples Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calculate the molar heat of enthalpy for a. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature. Explain About Calorimetry With Examples.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 Explain About Calorimetry With Examples Calculate the molar heat of enthalpy for a. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. In this article, we will. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. The. Explain About Calorimetry With Examples.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Explain About Calorimetry With Examples Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. One technique we can use to measure the amount of heat involved in a chemical or physical. Explain About Calorimetry With Examples.
From www.slideserve.com
PPT HEAT AND CALORIMETRY PowerPoint Presentation, free download ID4251428 Explain About Calorimetry With Examples In this article, we will. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. To do so, the heat is exchanged with a calibrated object. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry.. Explain About Calorimetry With Examples.
From www.youtube.com
09.05 Calorimetry YouTube Explain About Calorimetry With Examples Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. To do so, the heat is exchanged with a calibrated object. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. One technique we can use to measure the amount. Explain About Calorimetry With Examples.
From www.youtube.com
Physics 9.09g Calorimetry Example 2 YouTube Explain About Calorimetry With Examples Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. To do so, the heat is exchanged with a calibrated object. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released. Explain About Calorimetry With Examples.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Explain About Calorimetry With Examples In this article, we will. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. To do so, the heat is exchanged with a calibrated object. One technique we can. Explain About Calorimetry With Examples.
From www.youtube.com
Principle of Calorimetry YouTube Explain About Calorimetry With Examples Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. In this article, we will. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Explain About Calorimetry With Examples.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Explain About Calorimetry With Examples The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal. Explain About Calorimetry With Examples.
From saylordotorg.github.io
Calorimetry Explain About Calorimetry With Examples The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. One technique we can use to measure the amount of heat involved in a chemical or. Explain About Calorimetry With Examples.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID3207088 Explain About Calorimetry With Examples To do so, the heat is exchanged with a calibrated object. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry measures enthalpy changes during chemical processes, where the. Explain About Calorimetry With Examples.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Explain About Calorimetry With Examples The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal. Explain About Calorimetry With Examples.
From www.slideserve.com
PPT Calorimeters & Calorimetry PowerPoint Presentation, free download ID1611795 Explain About Calorimetry With Examples Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer. To do so, the heat is exchanged with a calibrated object. Calorimetry is a branch of science concerned with. Explain About Calorimetry With Examples.
From studylib.net
Calorimetry Explain About Calorimetry With Examples The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate. Explain About Calorimetry With Examples.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Explain About Calorimetry With Examples Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer. Calculate the molar heat of enthalpy for a. Calorimetry measures enthalpy changes during chemical processes, where. Explain About Calorimetry With Examples.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Explain About Calorimetry With Examples Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calculate the molar heat of enthalpy for a. Calorimetry is a branch of science concerned with measuring a body’s. Explain About Calorimetry With Examples.
From 2012books.lardbucket.org
Calorimetry Explain About Calorimetry With Examples One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. In this article, we will. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer. Calorimeters is an important chemistry lab instrument devices that measure the. Explain About Calorimetry With Examples.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types, Examples and Uses CollegeSearch Explain About Calorimetry With Examples Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. One technique we can use to measure the amount. Explain About Calorimetry With Examples.