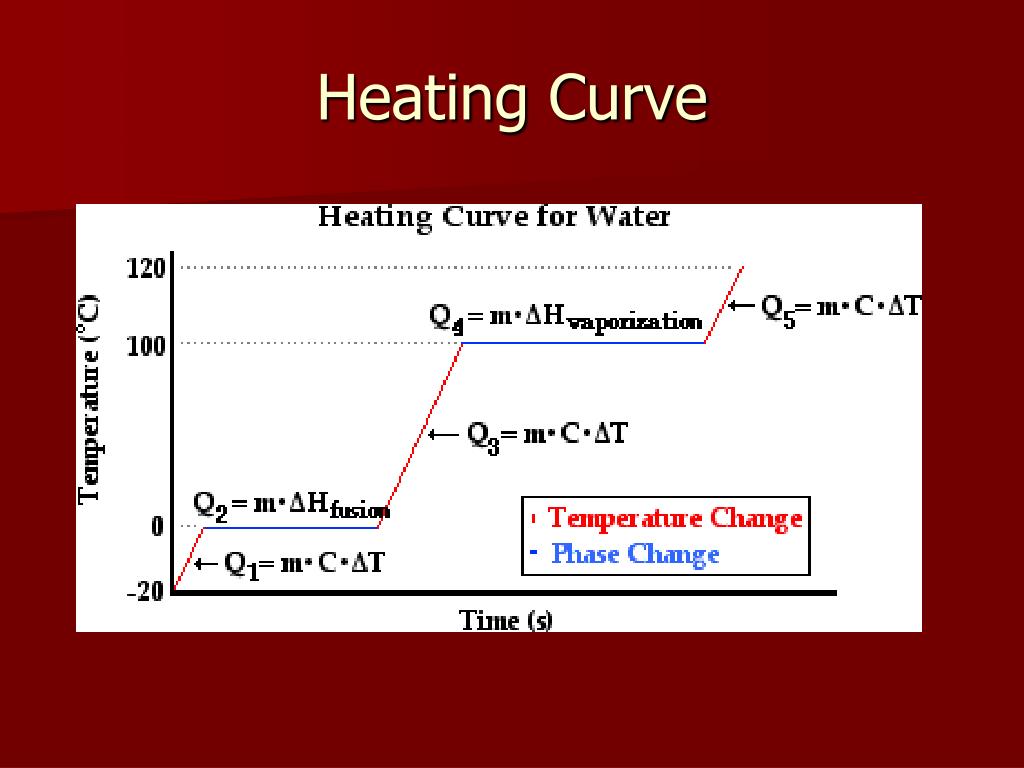
Heating Curve Equations . A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate). Learn how to correlate heating curves to phase diagrams and use phase diagrams to estimate conditions for phase transitions. Learn about the heating curve for water and how energy changes during phase transitions. Heat steam from 100 °c to 120 °c. The heat needed to change the temperature of a given substance (with no change in phase) is: The following equation allows us to calculate the amount of heat energy necessary to increase the temperature of a given mass (m) of substance by a certain amount (δt): Explore the enthalpy of vaporization and the clausius. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Learn how to interpret heating curves that show the temperature and heat flow of substances during heating or cooling processes. Explore the enthalpy of vaporization and the clausius. Learn how to correlate heating curves to phase diagrams and use phase diagrams to estimate conditions for phase transitions.
from www.slideserve.com
Explore the enthalpy of vaporization and the clausius. Learn how to correlate heating curves to phase diagrams and use phase diagrams to estimate conditions for phase transitions. Learn how to correlate heating curves to phase diagrams and use phase diagrams to estimate conditions for phase transitions. Heat steam from 100 °c to 120 °c. The following equation allows us to calculate the amount of heat energy necessary to increase the temperature of a given mass (m) of substance by a certain amount (δt): Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Learn how to interpret heating curves that show the temperature and heat flow of substances during heating or cooling processes. A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate). Learn about the heating curve for water and how energy changes during phase transitions. The heat needed to change the temperature of a given substance (with no change in phase) is:
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download ID6735511
Heating Curve Equations Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Learn how to correlate heating curves to phase diagrams and use phase diagrams to estimate conditions for phase transitions. A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate). Explore the enthalpy of vaporization and the clausius. Heat steam from 100 °c to 120 °c. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Learn how to correlate heating curves to phase diagrams and use phase diagrams to estimate conditions for phase transitions. Learn how to interpret heating curves that show the temperature and heat flow of substances during heating or cooling processes. The following equation allows us to calculate the amount of heat energy necessary to increase the temperature of a given mass (m) of substance by a certain amount (δt): Learn about the heating curve for water and how energy changes during phase transitions. Explore the enthalpy of vaporization and the clausius. The heat needed to change the temperature of a given substance (with no change in phase) is:
From www.slideshare.net
Energy ch 16 Heating Curve Equations Explore the enthalpy of vaporization and the clausius. Heat steam from 100 °c to 120 °c. Learn about the heating curve for water and how energy changes during phase transitions. The heat needed to change the temperature of a given substance (with no change in phase) is: Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time,. Heating Curve Equations.
From www.youtube.com
Heating Curve and Cooling Curve of Water Enthalpy of Fusion & Vaporization YouTube Heating Curve Equations Learn how to correlate heating curves to phase diagrams and use phase diagrams to estimate conditions for phase transitions. The heat needed to change the temperature of a given substance (with no change in phase) is: Learn about the heating curve for water and how energy changes during phase transitions. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature. Heating Curve Equations.
From studylib.net
Heat Equation Heating Curve Equations Heat steam from 100 °c to 120 °c. The heat needed to change the temperature of a given substance (with no change in phase) is: Explore the enthalpy of vaporization and the clausius. Learn how to correlate heating curves to phase diagrams and use phase diagrams to estimate conditions for phase transitions. Learn how to correlate heating curves to phase. Heating Curve Equations.
From chemistrytalk.org
Heat of Fusion Explained ChemTalk Heating Curve Equations Explore the enthalpy of vaporization and the clausius. The following equation allows us to calculate the amount of heat energy necessary to increase the temperature of a given mass (m) of substance by a certain amount (δt): Learn how to interpret heating curves that show the temperature and heat flow of substances during heating or cooling processes. The heat needed. Heating Curve Equations.
From www.expii.com
Heating and Cooling Curves — Overview & Examples Expii Heating Curve Equations Learn about the heating curve for water and how energy changes during phase transitions. Explore the enthalpy of vaporization and the clausius. The following equation allows us to calculate the amount of heat energy necessary to increase the temperature of a given mass (m) of substance by a certain amount (δt): Learn how to correlate heating curves to phase diagrams. Heating Curve Equations.
From chem.libretexts.org
5.5 Heating Curves and Phase Changes Chemistry LibreTexts Heating Curve Equations Learn how to correlate heating curves to phase diagrams and use phase diagrams to estimate conditions for phase transitions. Learn about the heating curve for water and how energy changes during phase transitions. Heat steam from 100 °c to 120 °c. Learn how to interpret heating curves that show the temperature and heat flow of substances during heating or cooling. Heating Curve Equations.
From www.youtube.com
Heating curve calculation (benzene) YouTube Heating Curve Equations Explore the enthalpy of vaporization and the clausius. Learn how to correlate heating curves to phase diagrams and use phase diagrams to estimate conditions for phase transitions. A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate). The following equation allows us to calculate the amount of heat energy. Heating Curve Equations.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation Heating Curve Equations Learn how to interpret heating curves that show the temperature and heat flow of substances during heating or cooling processes. Explore the enthalpy of vaporization and the clausius. Heat steam from 100 °c to 120 °c. The following equation allows us to calculate the amount of heat energy necessary to increase the temperature of a given mass (m) of substance. Heating Curve Equations.
From app.jove.com
Heating and Cooling Curves Concept Chemistry JoVe Heating Curve Equations The heat needed to change the temperature of a given substance (with no change in phase) is: Learn how to correlate heating curves to phase diagrams and use phase diagrams to estimate conditions for phase transitions. Learn how to interpret heating curves that show the temperature and heat flow of substances during heating or cooling processes. Explore the enthalpy of. Heating Curve Equations.
From www.youtube.com
AP Video 10.6 Intro to HeatingCooling Curves & Calculations YouTube Heating Curve Equations The heat needed to change the temperature of a given substance (with no change in phase) is: Learn how to correlate heating curves to phase diagrams and use phase diagrams to estimate conditions for phase transitions. Heat steam from 100 °c to 120 °c. Learn how to interpret heating curves that show the temperature and heat flow of substances during. Heating Curve Equations.
From www.youtube.com
Heating and Cooling Curve / Introduction plus and Potential Energy YouTube Heating Curve Equations The following equation allows us to calculate the amount of heat energy necessary to increase the temperature of a given mass (m) of substance by a certain amount (δt): Learn about the heating curve for water and how energy changes during phase transitions. Heat steam from 100 °c to 120 °c. Figure \(\pageindex{3}\) shows a heating curve, a plot of. Heating Curve Equations.
From www.youtube.com
Heating Curves Tutorial How to Calculate enthalpy changes in Heating & Cooling Crash Heating Curve Equations Learn about the heating curve for water and how energy changes during phase transitions. Learn how to correlate heating curves to phase diagrams and use phase diagrams to estimate conditions for phase transitions. A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate). Learn how to interpret heating curves. Heating Curve Equations.
From www.youtube.com
How to Read a Heating Curve YouTube Heating Curve Equations Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Learn how to correlate heating curves to phase diagrams and use phase diagrams to estimate conditions for phase transitions. Heat steam from 100 °c to 120 °c. The following equation allows us to calculate the amount of heat energy necessary. Heating Curve Equations.
From www.youtube.com
Heating Curve Calculation YouTube Heating Curve Equations Explore the enthalpy of vaporization and the clausius. Learn how to correlate heating curves to phase diagrams and use phase diagrams to estimate conditions for phase transitions. The following equation allows us to calculate the amount of heat energy necessary to increase the temperature of a given mass (m) of substance by a certain amount (δt): The heat needed to. Heating Curve Equations.
From www.slideserve.com
PPT Heating and Cooling Curves PowerPoint Presentation, free download ID2249930 Heating Curve Equations Explore the enthalpy of vaporization and the clausius. Explore the enthalpy of vaporization and the clausius. Heat steam from 100 °c to 120 °c. Learn how to correlate heating curves to phase diagrams and use phase diagrams to estimate conditions for phase transitions. Learn how to correlate heating curves to phase diagrams and use phase diagrams to estimate conditions for. Heating Curve Equations.
From www.youtube.com
Heating and Cooling Curves with Calculations YouTube Heating Curve Equations The heat needed to change the temperature of a given substance (with no change in phase) is: Explore the enthalpy of vaporization and the clausius. A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate). Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for. Heating Curve Equations.
From www.youtube.com
1617 Heating Curve Calculations YouTube Heating Curve Equations The following equation allows us to calculate the amount of heat energy necessary to increase the temperature of a given mass (m) of substance by a certain amount (δt): Explore the enthalpy of vaporization and the clausius. Learn how to correlate heating curves to phase diagrams and use phase diagrams to estimate conditions for phase transitions. Figure \(\pageindex{3}\) shows a. Heating Curve Equations.
From mungfali.com
Heat Transfer Coefficient Equation Heating Curve Equations Learn how to correlate heating curves to phase diagrams and use phase diagrams to estimate conditions for phase transitions. Learn how to correlate heating curves to phase diagrams and use phase diagrams to estimate conditions for phase transitions. The heat needed to change the temperature of a given substance (with no change in phase) is: Figure \(\pageindex{3}\) shows a heating. Heating Curve Equations.
From spmphysics.onlinetuition.com.my
The Heating Curve SPM Physics Form 4/Form 5 Revision Notes Heating Curve Equations Learn about the heating curve for water and how energy changes during phase transitions. Explore the enthalpy of vaporization and the clausius. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Explore the enthalpy of vaporization and the clausius. A heating curve of a substance shows the relationship of. Heating Curve Equations.
From chem.libretexts.org
5.5.1 Heating Curves and Phase Changes (Problems) Chemistry LibreTexts Heating Curve Equations The heat needed to change the temperature of a given substance (with no change in phase) is: The following equation allows us to calculate the amount of heat energy necessary to increase the temperature of a given mass (m) of substance by a certain amount (δt): Explore the enthalpy of vaporization and the clausius. Heat steam from 100 °c to. Heating Curve Equations.
From wisc.pb.unizin.org
M11Q2 Heating Curves and Phase Diagrams Chem 103/104 Resource Book Heating Curve Equations Explore the enthalpy of vaporization and the clausius. The heat needed to change the temperature of a given substance (with no change in phase) is: Explore the enthalpy of vaporization and the clausius. Heat steam from 100 °c to 120 °c. Learn how to correlate heating curves to phase diagrams and use phase diagrams to estimate conditions for phase transitions.. Heating Curve Equations.
From www.slideserve.com
PPT Heating Curve for Water PowerPoint Presentation, free download ID6790523 Heating Curve Equations Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The following equation allows us to calculate the amount of heat energy necessary to increase the temperature of a given mass (m) of substance by a certain amount (δt): A heating curve of a substance shows the relationship of temperature,. Heating Curve Equations.
From socratic.org
What are the 6 phase changes along a heating curve? Socratic Heating Curve Equations Explore the enthalpy of vaporization and the clausius. Explore the enthalpy of vaporization and the clausius. A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate). Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The following equation. Heating Curve Equations.
From ch301.cm.utexas.edu
heating curve Heating Curve Equations A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate). Learn how to interpret heating curves that show the temperature and heat flow of substances during heating or cooling processes. Learn about the heating curve for water and how energy changes during phase transitions. Heat steam from 100 °c. Heating Curve Equations.
From www.worldwisetutoring.com
Heating and Cooling Curves Heating Curve Equations Heat steam from 100 °c to 120 °c. Learn how to interpret heating curves that show the temperature and heat flow of substances during heating or cooling processes. The following equation allows us to calculate the amount of heat energy necessary to increase the temperature of a given mass (m) of substance by a certain amount (δt): Explore the enthalpy. Heating Curve Equations.
From studylib.net
A.2 Heat Curves Phase diagram Worksheet Key Heating Curve Equations Heat steam from 100 °c to 120 °c. Explore the enthalpy of vaporization and the clausius. Explore the enthalpy of vaporization and the clausius. A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate). Learn about the heating curve for water and how energy changes during phase transitions. The. Heating Curve Equations.
From www.youtube.com
Heating Curves and Energy Calculations YouTube Heating Curve Equations Heat steam from 100 °c to 120 °c. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Learn how to interpret heating curves that show the temperature and heat flow of substances during heating or cooling processes. A heating curve of a substance shows the relationship of temperature, state. Heating Curve Equations.
From materiallistgaskell.z21.web.core.windows.net
Heating Curve With Equations Heating Curve Equations Explore the enthalpy of vaporization and the clausius. Learn about the heating curve for water and how energy changes during phase transitions. A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate). Learn how to interpret heating curves that show the temperature and heat flow of substances during heating. Heating Curve Equations.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download ID6735511 Heating Curve Equations Learn about the heating curve for water and how energy changes during phase transitions. Learn how to correlate heating curves to phase diagrams and use phase diagrams to estimate conditions for phase transitions. The following equation allows us to calculate the amount of heat energy necessary to increase the temperature of a given mass (m) of substance by a certain. Heating Curve Equations.
From www.youtube.com
HEATING CURVE How to Read & How TO Draw A Heating Curve [ AboodyTV ] Chemistry YouTube Heating Curve Equations Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Learn how to correlate heating curves to phase diagrams and use phase diagrams to estimate conditions for phase transitions. The following equation allows us to calculate the amount of heat energy necessary to increase the temperature of a given mass. Heating Curve Equations.
From www.pinterest.com
Heating curve calculation (benzene) Worksheets, Printable preschool worksheets, Chemistry Heating Curve Equations Explore the enthalpy of vaporization and the clausius. The heat needed to change the temperature of a given substance (with no change in phase) is: Heat steam from 100 °c to 120 °c. Learn how to interpret heating curves that show the temperature and heat flow of substances during heating or cooling processes. A heating curve of a substance shows. Heating Curve Equations.
From www.slideserve.com
PPT Thermal Properties of Matter (Part I) PowerPoint Presentation, free download ID5077863 Heating Curve Equations Heat steam from 100 °c to 120 °c. Learn about the heating curve for water and how energy changes during phase transitions. Explore the enthalpy of vaporization and the clausius. The heat needed to change the temperature of a given substance (with no change in phase) is: Learn how to correlate heating curves to phase diagrams and use phase diagrams. Heating Curve Equations.
From www.chegg.com
Solved The graph above shows the heating curve of water. One Heating Curve Equations Learn how to correlate heating curves to phase diagrams and use phase diagrams to estimate conditions for phase transitions. The following equation allows us to calculate the amount of heat energy necessary to increase the temperature of a given mass (m) of substance by a certain amount (δt): Explore the enthalpy of vaporization and the clausius. A heating curve of. Heating Curve Equations.
From preparatorychemistry.com
Heating Curve Heating Curve Equations The following equation allows us to calculate the amount of heat energy necessary to increase the temperature of a given mass (m) of substance by a certain amount (δt): Learn how to correlate heating curves to phase diagrams and use phase diagrams to estimate conditions for phase transitions. Explore the enthalpy of vaporization and the clausius. Learn how to interpret. Heating Curve Equations.
From www.youtube.com
What Does It Mean to Solve the Heat Equation PDE? An Introduction with Example Solutions and Heating Curve Equations The heat needed to change the temperature of a given substance (with no change in phase) is: Learn about the heating curve for water and how energy changes during phase transitions. Heat steam from 100 °c to 120 °c. The following equation allows us to calculate the amount of heat energy necessary to increase the temperature of a given mass. Heating Curve Equations.