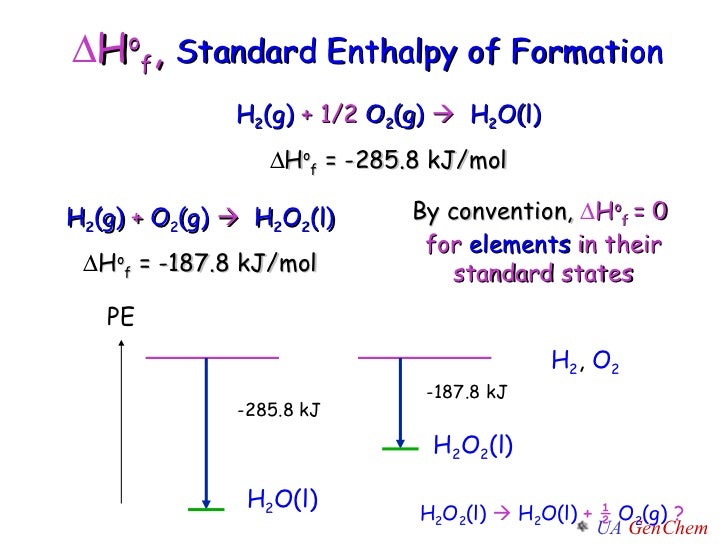
Standard Enthalpy Of Formation H2O Gas . Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: All standard state, 25 °c and 1 bar (written to 1 decimal place). Mallard, eds, nist chemistry webbook, nist standard reference database. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard.
from www.slideshare.net
This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. All standard state, 25 °c and 1 bar (written to 1 decimal place). Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: Mallard, eds, nist chemistry webbook, nist standard reference database. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and.
Lect w10 abbrev_ thermochemistry_alg
Standard Enthalpy Of Formation H2O Gas 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Mallard, eds, nist chemistry webbook, nist standard reference database. A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: All standard state, 25 °c and 1 bar (written to 1 decimal place). By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and.
From cewuaeqb.blob.core.windows.net
How Do You Work Out Standard Enthalpy Of Formation at Connie Stroud blog Standard Enthalpy Of Formation H2O Gas A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard. Standard Enthalpy Of Formation H2O Gas.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation H2O Gas Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: All standard state, 25 °c and 1 bar (written to 1 decimal place). A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard. Standard Enthalpy Of Formation H2O Gas.
From www.chegg.com
Solved 13. The standard enthalpies of formation for several Standard Enthalpy Of Formation H2O Gas All standard state, 25 °c and 1 bar (written to 1 decimal place). A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. By definition, the standard enthalpy. Standard Enthalpy Of Formation H2O Gas.
From joilylugg.blob.core.windows.net
Standard Enthalpy Of Formation Def at Sandra Leonard blog Standard Enthalpy Of Formation H2O Gas Mallard, eds, nist chemistry webbook, nist standard reference database. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. By definition, the standard enthalpy of formation. Standard Enthalpy Of Formation H2O Gas.
From www.nagwa.com
Question Video Calculating the Standard Enthalpy of Formation for Heptane Using Standard Standard Enthalpy Of Formation H2O Gas Mallard, eds, nist chemistry webbook, nist standard reference database. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. All standard state,. Standard Enthalpy Of Formation H2O Gas.
From pdfprof.com
enthalpies standard de formation et entropie standard Standard Enthalpy Of Formation H2O Gas This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: The standard enthalpy of formation is. Standard Enthalpy Of Formation H2O Gas.
From www.toppr.com
The standard enthalpy of formation of H2O (l) and Fe2O3 (s) are respectively 286 kJ mol^1 and Standard Enthalpy Of Formation H2O Gas Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. By definition, the standard enthalpy of formation of an element in its most stable form is equal. Standard Enthalpy Of Formation H2O Gas.
From cepbtpfh.blob.core.windows.net
Standard Heat Of Formation Hydrogen Peroxide at John Ahmed blog Standard Enthalpy Of Formation H2O Gas Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Mallard, eds, nist chemistry webbook,. Standard Enthalpy Of Formation H2O Gas.
From oxygengasnaraeru.blogspot.com
Oxygen Gas Enthalpy Of Formation Of Oxygen Gas Standard Enthalpy Of Formation H2O Gas The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. All standard state, 25 °c and 1 bar (written to 1 decimal place). This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the.. Standard Enthalpy Of Formation H2O Gas.
From cepbtpfh.blob.core.windows.net
Standard Heat Of Formation Hydrogen Peroxide at John Ahmed blog Standard Enthalpy Of Formation H2O Gas Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. 136 rows standard enthalpy change of. Standard Enthalpy Of Formation H2O Gas.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Change YouTube Standard Enthalpy Of Formation H2O Gas This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. By definition, the standard enthalpy of formation of an element in its most stable form. Standard Enthalpy Of Formation H2O Gas.
From www.slideshare.net
Lect w10 abbrev_ thermochemistry_alg Standard Enthalpy Of Formation H2O Gas The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Mallard, eds, nist chemistry webbook, nist standard reference database. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for.. Standard Enthalpy Of Formation H2O Gas.
From www.numerade.com
SOLVED Calculate the standard enthalpy of combustion for propane gas in kj/mol. the standard Standard Enthalpy Of Formation H2O Gas Mallard, eds, nist chemistry webbook, nist standard reference database. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with. Definition and explanation of the terms standard state and. Standard Enthalpy Of Formation H2O Gas.
From www.numerade.com
SOLVED Using the standard enthalpies of formation, what is the standard enthalpy of reaction Standard Enthalpy Of Formation H2O Gas 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. All standard state, 25 °c and 1 bar (written to 1 decimal place). Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. The standard enthalpy. Standard Enthalpy Of Formation H2O Gas.
From www.numerade.com
SOLVED6. Calculate the standard enthalpy change (AH) for the combustion reaction of ethanol Standard Enthalpy Of Formation H2O Gas This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: Mallard, eds, nist chemistry webbook, nist standard reference database. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from. Standard Enthalpy Of Formation H2O Gas.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Enthalpy Of Formation H2O Gas By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A pressure of 1 atm for gases and a concentration. Standard Enthalpy Of Formation H2O Gas.
From byjus.com
21. At 300 K standard enthalpy of formation of C6H5COOH(s), CO2(g), and H2O(l) are 408, 393, and Standard Enthalpy Of Formation H2O Gas Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: All standard state, 25 °c and 1 bar (written to 1 decimal place). A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with. Mallard, eds, nist chemistry webbook, nist standard reference database. Definition and explanation of the terms standard state and standard. Standard Enthalpy Of Formation H2O Gas.
From www.toppr.com
89. If standard molar enthalpy of formation of carbon dioxide gas, liquid water and ethane gas Standard Enthalpy Of Formation H2O Gas Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A pressure of 1 atm for gases and a concentration of 1 m for species in. Standard Enthalpy Of Formation H2O Gas.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all answers to three sig figs Standard Enthalpy Of Formation H2O Gas 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: Mallard, eds, nist chemistry. Standard Enthalpy Of Formation H2O Gas.
From www.nagwa.com
Question Video Calculating the Standard Heat of Reaction for the Combustion of Methane Using Standard Enthalpy Of Formation H2O Gas A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with. All standard state, 25 °c and 1 bar (written to 1 decimal place). Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero. Standard Enthalpy Of Formation H2O Gas.
From www.numerade.com
Consider the following reaction 2 H2S (g) + 3 O2 (g) → 2 SO2 (g) + 2 H2O (g) Calculate the Standard Enthalpy Of Formation H2O Gas Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Mallard, eds, nist chemistry webbook, nist standard reference database. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under. Standard Enthalpy Of Formation H2O Gas.
From www.chegg.com
Solved Using the standard enthalpies of formation listed in Standard Enthalpy Of Formation H2O Gas 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Mallard, eds, nist chemistry webbook, nist standard reference database. All standard state, 25 °c and. Standard Enthalpy Of Formation H2O Gas.
From www.youtube.com
5.1 Standard enthalpy changes of formation and combustion YouTube Standard Enthalpy Of Formation H2O Gas 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Mallard, eds, nist chemistry webbook, nist standard reference database. Standard enthalpies of formation (\(δh^o_{f}\)). Standard Enthalpy Of Formation H2O Gas.
From cewuaeqb.blob.core.windows.net
How Do You Work Out Standard Enthalpy Of Formation at Connie Stroud blog Standard Enthalpy Of Formation H2O Gas All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the.. Standard Enthalpy Of Formation H2O Gas.
From chem.libretexts.org
5.7 Enthalpies of Formation Chemistry LibreTexts Standard Enthalpy Of Formation H2O Gas This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Definition and explanation of the terms standard state and standard enthalpy of formation, with. Standard Enthalpy Of Formation H2O Gas.
From www.numerade.com
SOLVED (b) Cars burn gasoline as fuel according to the equation C8H18() 02(g) CO2(g) H2O(g Standard Enthalpy Of Formation H2O Gas This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing. Standard Enthalpy Of Formation H2O Gas.
From nasirghopbuck.blogspot.com
How to Calculate the Standard Enthalpy of Combustion Standard Enthalpy Of Formation H2O Gas 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. This table lists the standard enthalpies (δh°), the free energies. Standard Enthalpy Of Formation H2O Gas.
From www.slideserve.com
PPT Hess's Law PowerPoint Presentation, free download ID3316379 Standard Enthalpy Of Formation H2O Gas This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Mallard, eds, nist chemistry webbook, nist standard reference database. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Definition and explanation of the. Standard Enthalpy Of Formation H2O Gas.
From www.researchgate.net
Enthalpy of formation and heats of formation of main combustion... Download Scientific Diagram Standard Enthalpy Of Formation H2O Gas A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. Standard Enthalpy Of Formation H2O Gas.
From hydrogengasgaosube.blogspot.com
Hydrogen Gas June 2017 Standard Enthalpy Of Formation H2O Gas By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Definition and explanation of the terms standard state and. Standard Enthalpy Of Formation H2O Gas.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation H2O Gas By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. All standard. Standard Enthalpy Of Formation H2O Gas.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free download ID228912 Standard Enthalpy Of Formation H2O Gas Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Mallard, eds, nist chemistry webbook, nist standard reference database. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero. Standard Enthalpy Of Formation H2O Gas.
From www.chegg.com
Solved The standard enthalpy of formation of gaseous H2O at Standard Enthalpy Of Formation H2O Gas This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard. Standard Enthalpy Of Formation H2O Gas.
From www.chegg.com
Solved The standard enthalpy of formation of H2O(g) at 298 K Standard Enthalpy Of Formation H2O Gas 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. All standard state, 25 °c and 1 bar (written to 1 decimal place). 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Mallard, eds,. Standard Enthalpy Of Formation H2O Gas.
From www.numerade.com
(a) Calculate the standard enthalpy of formation of gaseous diborane (B2 H6) using the following Standard Enthalpy Of Formation H2O Gas Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with. Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: 136 rows standard enthalpy change of formation (data table) these tables. Standard Enthalpy Of Formation H2O Gas.