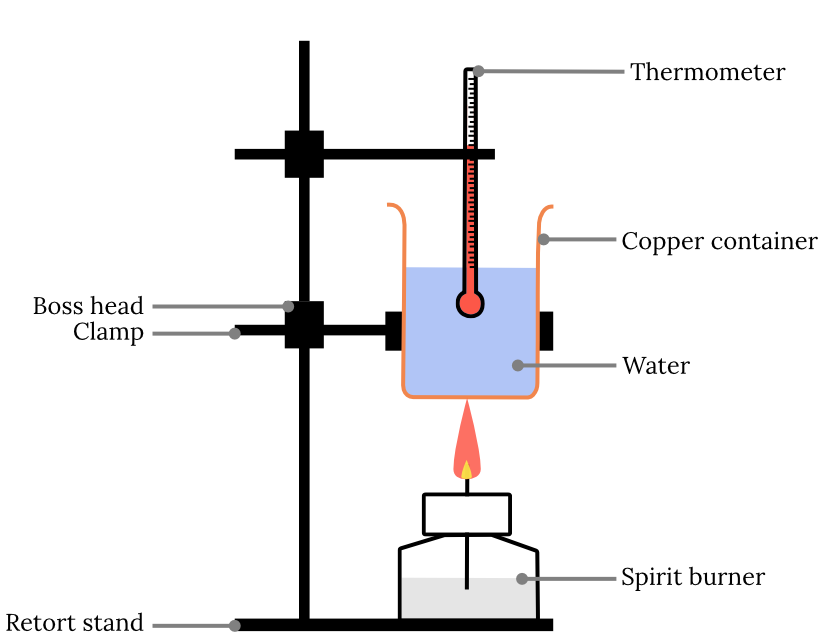
Calorimeter Experiment Analysis . Measuring the latent heat of fusion of water, and measuring the specific heat capacities. a calorimeter is a device used to determine heat flow during a chemical or physical change. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside. To do so, the heat is exchanged with a. in this lab, you will do two classic calorimetry experiments: calorimetry is used to measure amounts of heat transferred to or from a substance. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed.
from www.learnable.education
calorimetry is used to measure amounts of heat transferred to or from a substance. Measuring the latent heat of fusion of water, and measuring the specific heat capacities. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside. calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. a calorimeter is a device used to determine heat flow during a chemical or physical change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To do so, the heat is exchanged with a. For example, when an exothermic reaction. in this lab, you will do two classic calorimetry experiments:
Year 11 Chemistry Practical Investigation Calorimetry Experiment
Calorimeter Experiment Analysis calorimetry is used to measure amounts of heat transferred to or from a substance. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside. a calorimeter is a device used to determine heat flow during a chemical or physical change. Measuring the latent heat of fusion of water, and measuring the specific heat capacities. in this lab, you will do two classic calorimetry experiments: For example, when an exothermic reaction. To do so, the heat is exchanged with a. calorimetry is used to measure amounts of heat transferred to or from a substance.
From www.youtube.com
How to Draw a Calorimeter Step by Step Drawing Tutorial YouTube Calorimeter Experiment Analysis calorimetry is used to measure amounts of heat transferred to or from a substance. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. . Calorimeter Experiment Analysis.
From exozspczc.blob.core.windows.net
Calorimeter Experiment Introduction at Jose Evans blog Calorimeter Experiment Analysis in this lab, you will do two classic calorimetry experiments: For example, when an exothermic reaction. To do so, the heat is exchanged with a. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed.. Calorimeter Experiment Analysis.
From cider.uoregon.edu
Heat of Solution Calorimetry Simulation Dissolving Salts in Water CIDER Calorimeter Experiment Analysis Measuring the latent heat of fusion of water, and measuring the specific heat capacities. To do so, the heat is exchanged with a. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside. a calorimeter is a device used to determine heat flow during a. Calorimeter Experiment Analysis.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter Experiment Analysis calorimetry is used to measure amounts of heat transferred to or from a substance. For example, when an exothermic reaction. calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. a calorimeter is a device used to determine heat flow during a chemical or physical change. To do so,. Calorimeter Experiment Analysis.
From www.slideserve.com
PPT Chapter 12 “Heat in Chemical Reactions” PowerPoint Presentation ID2132588 Calorimeter Experiment Analysis To do so, the heat is exchanged with a. in this lab, you will do two classic calorimetry experiments: calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. Measuring the latent heat of fusion of water, and measuring the specific heat capacities. calorimetry is used to measure amounts. Calorimeter Experiment Analysis.
From studylib.net
experiment 9 Thermochemistry Calorimetry Calorimeter Experiment Analysis Measuring the latent heat of fusion of water, and measuring the specific heat capacities. a calorimeter is a device used to determine heat flow during a chemical or physical change. calorimetry is used to measure amounts of heat transferred to or from a substance. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot. Calorimeter Experiment Analysis.
From youtube.com
Heat of Reaction (Calorimetry) Experiment YouTube Calorimeter Experiment Analysis calorimetry is used to measure amounts of heat transferred to or from a substance. in this lab, you will do two classic calorimetry experiments: a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To do so, the heat is exchanged with a. a calorimeter is a. Calorimeter Experiment Analysis.
From courses.lumenlearning.com
Calorimetry Chemistry I Calorimeter Experiment Analysis experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside. To do so, the heat is exchanged with a. calorimetry is used to measure amounts of heat transferred to or from a substance. in this lab, you will do two classic calorimetry experiments: For. Calorimeter Experiment Analysis.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 3.2 Describe Simple Calorimetry Experiments for Reactions such as Calorimeter Experiment Analysis Measuring the latent heat of fusion of water, and measuring the specific heat capacities. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to determine heat flow during a chemical or physical change. calorimetry is based on the first law of. Calorimeter Experiment Analysis.
From www.researchgate.net
Temperature measurements during a typical calorimetry experiment Download Scientific Diagram Calorimeter Experiment Analysis a calorimeter is a device used to determine heat flow during a chemical or physical change. For example, when an exothermic reaction. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a. calorimetry is based on the first law of thermodynamics that states that. Calorimeter Experiment Analysis.
From studyfinder.org
Unveiling the Secrets of Experiment 25 Calorimetry Prelab Answers Revealed Calorimeter Experiment Analysis a calorimeter is a device used to determine heat flow during a chemical or physical change. in this lab, you will do two classic calorimetry experiments: calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. To do so, the heat is exchanged with a. For example, when an. Calorimeter Experiment Analysis.
From www.researchgate.net
Basic principle of isothermal titration calorimetry. Schematic... Download Scientific Diagram Calorimeter Experiment Analysis To do so, the heat is exchanged with a. calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside. a calorimeter is a device used to. Calorimeter Experiment Analysis.
From cerifsbe.blob.core.windows.net
Calorimetry Fuels Experiment at Francis Edler blog Calorimeter Experiment Analysis a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. in this lab, you will do two classic calorimetry experiments: experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water. Calorimeter Experiment Analysis.
From www.linstitute.net
IB DP Chemistry HL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 Calorimeter Experiment Analysis a calorimeter is a device used to determine heat flow during a chemical or physical change. in this lab, you will do two classic calorimetry experiments: a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity. Calorimeter Experiment Analysis.
From exonegtdm.blob.core.windows.net
A Level Chemistry Calorimetry Practical Aqa at Richelle Doty blog Calorimeter Experiment Analysis a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Measuring the latent heat of fusion of water, and measuring the specific heat capacities. in this lab, you will do two classic calorimetry experiments: a calorimeter is a device used to determine heat flow during a chemical or. Calorimeter Experiment Analysis.
From www.pinterest.com
Calorimetry Lab Report Science diagrams, Thermodynamics, How to find out Calorimeter Experiment Analysis calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. a calorimeter is a device used to determine heat flow during a chemical or physical change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. in this lab,. Calorimeter Experiment Analysis.
From users.highland.edu
Calorimetry Calorimeter Experiment Analysis in this lab, you will do two classic calorimetry experiments: calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. a calorimeter is a device used to measure the amount of heat involved in. Calorimeter Experiment Analysis.
From studylib.net
Calorimetry Lab Specific Heat Capacity Calorimeter Experiment Analysis Measuring the latent heat of fusion of water, and measuring the specific heat capacities. calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. For example, when an exothermic reaction. To do so, the heat is exchanged with a. calorimetry is used to measure amounts of heat transferred to or. Calorimeter Experiment Analysis.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle and Structure of Calorimeter Experiment Analysis For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed.. Calorimeter Experiment Analysis.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Calorimeter Experiment Analysis a calorimeter is a device used to determine heat flow during a chemical or physical change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. in this lab, you will do two classic calorimetry experiments: To do so, the heat is exchanged with a. For example, when. Calorimeter Experiment Analysis.
From answermagicmatney.z21.web.core.windows.net
Coffee Cup Calorimetry Experiment Calorimeter Experiment Analysis Measuring the latent heat of fusion of water, and measuring the specific heat capacities. For example, when an exothermic reaction. a calorimeter is a device used to determine heat flow during a chemical or physical change. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water. Calorimeter Experiment Analysis.
From www.studocu.com
Calorimetry Lab Report Write an abstract for the calorimetry experiment. An example abstract Calorimeter Experiment Analysis To do so, the heat is exchanged with a. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside. in this lab, you will do. Calorimeter Experiment Analysis.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimeter Experiment Analysis For example, when an exothermic reaction. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside. in this lab, you will do two classic calorimetry experiments: calorimetry is used to measure amounts of heat transferred to or from a substance. a calorimeter is. Calorimeter Experiment Analysis.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Calorimeters Calorimeter Experiment Analysis To do so, the heat is exchanged with a. Measuring the latent heat of fusion of water, and measuring the specific heat capacities. For example, when an exothermic reaction. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside. in this lab, you will do. Calorimeter Experiment Analysis.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Calorimeter Experiment Analysis a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. To do so, the heat is exchanged with a. calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. experiment 6 ∙ calorimetry 6‐3. Calorimeter Experiment Analysis.
From www.education.com
Calorimetry Bomb Calorimeter Experiment Calorimeter Experiment Analysis To do so, the heat is exchanged with a. a calorimeter is a device used to determine heat flow during a chemical or physical change. Measuring the latent heat of fusion of water, and measuring the specific heat capacities. For example, when an exothermic reaction. calorimetry is used to measure amounts of heat transferred to or from a. Calorimeter Experiment Analysis.
From www.researchgate.net
Reaction calorimeter used for the experiments Download Scientific Diagram Calorimeter Experiment Analysis a calorimeter is a device used to determine heat flow during a chemical or physical change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water. Calorimeter Experiment Analysis.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimeter Experiment Analysis calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. in this lab, you will do two classic calorimetry experiments: To do so, the heat is exchanged with a. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in. Calorimeter Experiment Analysis.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Review for Exams YouTube Calorimeter Experiment Analysis in this lab, you will do two classic calorimetry experiments: a calorimeter is a device used to determine heat flow during a chemical or physical change. calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. a calorimeter is a device used to measure the amount of heat. Calorimeter Experiment Analysis.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow (M6Q5) UWMadison Calorimeter Experiment Analysis a calorimeter is a device used to determine heat flow during a chemical or physical change. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. Measuring the latent heat of fusion of water, and. Calorimeter Experiment Analysis.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimeter Experiment Analysis a calorimeter is a device used to determine heat flow during a chemical or physical change. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside. For example, when an exothermic reaction. in this lab, you will do two classic calorimetry experiments: calorimetry. Calorimeter Experiment Analysis.
From 2012books.lardbucket.org
Calorimetry Calorimeter Experiment Analysis calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. a calorimeter is a device used to determine heat flow during a chemical or physical change. Measuring the latent heat of fusion of water, and measuring the specific heat capacities. To do so, the heat is exchanged with a. . Calorimeter Experiment Analysis.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimeter Experiment Analysis a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Measuring the latent heat of fusion of water, and measuring the specific heat capacities. calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. For example, when an exothermic reaction. To. Calorimeter Experiment Analysis.
From martinfersbanks.blogspot.com
Is a Bomb Calorimeter Constant Pressure Calorimeter Experiment Analysis a calorimeter is a device used to determine heat flow during a chemical or physical change. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is based. Calorimeter Experiment Analysis.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimeter Experiment Analysis Measuring the latent heat of fusion of water, and measuring the specific heat capacities. calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. calorimetry is used to measure amounts of heat transferred to or from a substance. a calorimeter is a device used to measure the amount of. Calorimeter Experiment Analysis.