Master Record Production . in this post, we’ll show you how to prepare a batch manufacturing record, walk you through the benefits and features to look for in a new system and even. a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. § 225.102 master record file and production records. a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. (a) the master record file provides the complete procedure for manufacturing a. a master batch record (mbr) contains the instructions, recipe or formula, and specific manufacturing process for a particular. in cgmp manufacturing, all manufacturers are required to keep master batch records (mbrs) and batch production records (bprs) in order to comply with fda regulations. in pharmaceutical manufacturing, one document holds paramount importance in ensuring compliance and guaranteeing product quality — the master batch record. Master batch records, also known as master manufacturing formulas, are general manufacturing instructions.
from www.arenasolutions.com
a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. in this post, we’ll show you how to prepare a batch manufacturing record, walk you through the benefits and features to look for in a new system and even. Master batch records, also known as master manufacturing formulas, are general manufacturing instructions. a master batch record (mbr) contains the instructions, recipe or formula, and specific manufacturing process for a particular. in cgmp manufacturing, all manufacturers are required to keep master batch records (mbrs) and batch production records (bprs) in order to comply with fda regulations. in pharmaceutical manufacturing, one document holds paramount importance in ensuring compliance and guaranteeing product quality — the master batch record. § 225.102 master record file and production records. (a) the master record file provides the complete procedure for manufacturing a. a master batch record (mbr), also known as a master production record (mpr) is a document that contains the.
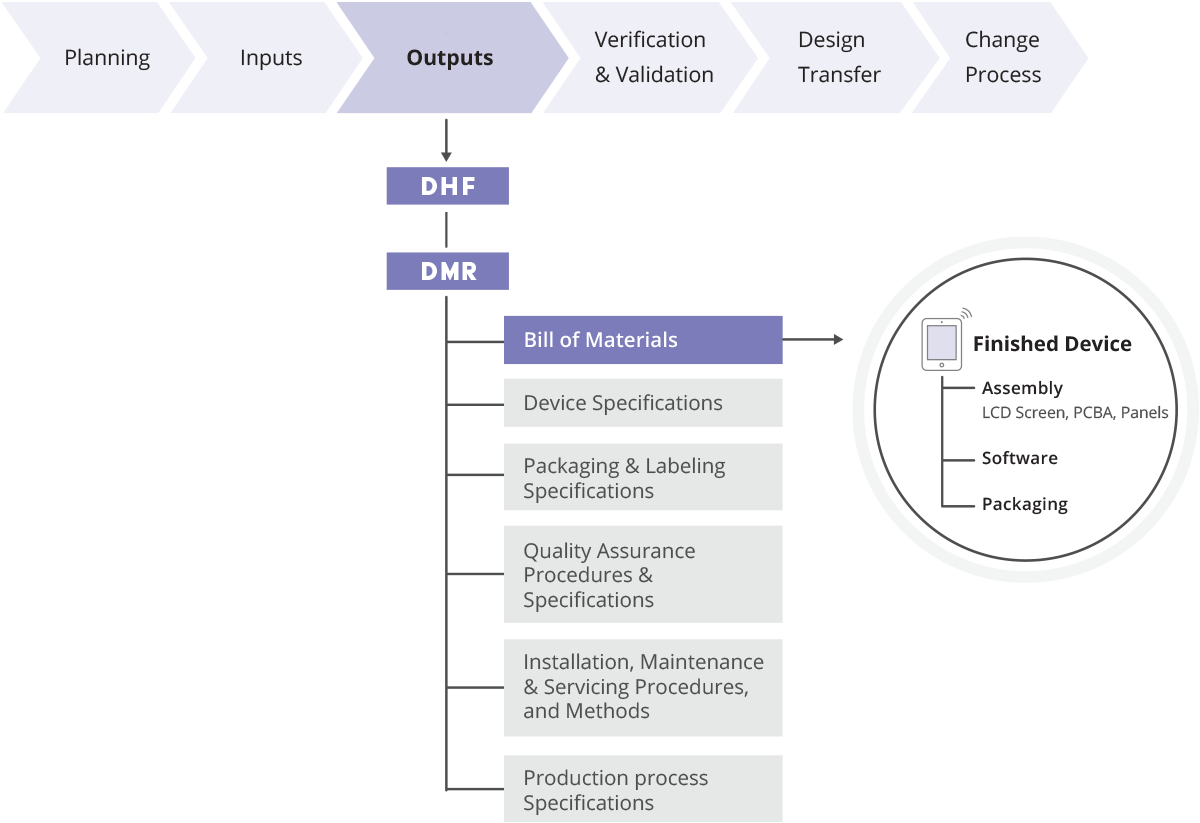
Device Master Record (DMR) Definition Arena
Master Record Production Master batch records, also known as master manufacturing formulas, are general manufacturing instructions. in this post, we’ll show you how to prepare a batch manufacturing record, walk you through the benefits and features to look for in a new system and even. Master batch records, also known as master manufacturing formulas, are general manufacturing instructions. (a) the master record file provides the complete procedure for manufacturing a. a master batch record (mbr) contains the instructions, recipe or formula, and specific manufacturing process for a particular. in pharmaceutical manufacturing, one document holds paramount importance in ensuring compliance and guaranteeing product quality — the master batch record. a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. § 225.102 master record file and production records. in cgmp manufacturing, all manufacturers are required to keep master batch records (mbrs) and batch production records (bprs) in order to comply with fda regulations. a master batch record (mbr), also known as a master production record (mpr) is a document that contains the.
From www.alamy.com
Making a negative of the master. Record Industry Vinyl Factory, Haarlem, Netherlands. Architect Master Record Production (a) the master record file provides the complete procedure for manufacturing a. a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. in pharmaceutical manufacturing, one document holds paramount importance in ensuring compliance and guaranteeing product quality — the master batch record. a master batch record (mbr), also. Master Record Production.
From www.slideshare.net
Master batch record,batch production record ,Quality Audit Type and p… Master Record Production in pharmaceutical manufacturing, one document holds paramount importance in ensuring compliance and guaranteeing product quality — the master batch record. in cgmp manufacturing, all manufacturers are required to keep master batch records (mbrs) and batch production records (bprs) in order to comply with fda regulations. a master batch record (mbr), also known as a master production record. Master Record Production.
From www.mastercontrol.com
Master Production Records MasterControl Master Record Production § 225.102 master record file and production records. a master batch record (mbr) contains the instructions, recipe or formula, and specific manufacturing process for a particular. a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. in cgmp manufacturing, all manufacturers are required to keep master batch. Master Record Production.
From www.instantgmp.com
Master Production Record Feature from InstantGMP Master Record Production § 225.102 master record file and production records. a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. in pharmaceutical manufacturing, one document holds paramount importance in ensuring compliance and guaranteeing product quality — the master batch record. in this post, we’ll show you how to prepare. Master Record Production.
From www.slideshare.net
Master batch record,batch production record ,Quality Audit Type and p… Master Record Production a master batch record (mbr) contains the instructions, recipe or formula, and specific manufacturing process for a particular. Master batch records, also known as master manufacturing formulas, are general manufacturing instructions. in cgmp manufacturing, all manufacturers are required to keep master batch records (mbrs) and batch production records (bprs) in order to comply with fda regulations. a. Master Record Production.
From www.facebook.com
How Vinyl Records Are Made ⚫️ phonograph record, record producer Learn how vinyl records are Master Record Production in this post, we’ll show you how to prepare a batch manufacturing record, walk you through the benefits and features to look for in a new system and even. (a) the master record file provides the complete procedure for manufacturing a. § 225.102 master record file and production records. in pharmaceutical manufacturing, one document holds paramount importance. Master Record Production.
From www.instantgmp.com
Batch Master Production Record Manufacturing Formula Master Record Production Master batch records, also known as master manufacturing formulas, are general manufacturing instructions. (a) the master record file provides the complete procedure for manufacturing a. a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. in this post, we’ll show you how to prepare a batch manufacturing record, walk. Master Record Production.
From templates.rjuuc.edu.np
Master Manufacturing Record Template Master Record Production in pharmaceutical manufacturing, one document holds paramount importance in ensuring compliance and guaranteeing product quality — the master batch record. a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. (a) the master record file provides the complete procedure for manufacturing a. in this post, we’ll show you. Master Record Production.
From www.youtube.com
SAP Production Planning (PP) Step 01 Change Material Master Record Mac OS YouTube Master Record Production a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. § 225.102 master record file and production records. in pharmaceutical manufacturing, one document holds paramount importance in ensuring compliance and guaranteeing product quality — the master batch record. in this post, we’ll show you how to prepare. Master Record Production.
From www.solutionspile.com
[Solved] Complete the following master schedule record. Master Record Production Master batch records, also known as master manufacturing formulas, are general manufacturing instructions. a master batch record (mbr) contains the instructions, recipe or formula, and specific manufacturing process for a particular. a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. in cgmp manufacturing, all manufacturers are required. Master Record Production.
From www.slideserve.com
PPT Batch Manufacturing Record and Master Formula Record PowerPoint Presentation ID12046824 Master Record Production in cgmp manufacturing, all manufacturers are required to keep master batch records (mbrs) and batch production records (bprs) in order to comply with fda regulations. Master batch records, also known as master manufacturing formulas, are general manufacturing instructions. a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. . Master Record Production.
From www.musicproductionglossary.com
Music Producer What is a music producer Master Record Production a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. a master batch record (mbr) contains the instructions, recipe or formula, and specific manufacturing process for a particular. . Master Record Production.
From www.chegg.com
Solved Complete the Master Production Schedule (MPS) record Master Record Production (a) the master record file provides the complete procedure for manufacturing a. § 225.102 master record file and production records. in pharmaceutical manufacturing, one document holds paramount importance in ensuring compliance and guaranteeing product quality — the master batch record. in cgmp manufacturing, all manufacturers are required to keep master batch records (mbrs) and batch production records. Master Record Production.
From rf-seoul.com
Music Production Record Factory Seoul Master Record Production in pharmaceutical manufacturing, one document holds paramount importance in ensuring compliance and guaranteeing product quality — the master batch record. in this post, we’ll show you how to prepare a batch manufacturing record, walk you through the benefits and features to look for in a new system and even. § 225.102 master record file and production records.. Master Record Production.
From cemhadnp.blob.core.windows.net
Master Batch Record 21 Cfr at Jacqueline Sadler blog Master Record Production § 225.102 master record file and production records. (a) the master record file provides the complete procedure for manufacturing a. in cgmp manufacturing, all manufacturers are required to keep master batch records (mbrs) and batch production records (bprs) in order to comply with fda regulations. in pharmaceutical manufacturing, one document holds paramount importance in ensuring compliance and. Master Record Production.
From www.vrogue.co
Master Production Schedule Excel Template Vrogue Master Record Production (a) the master record file provides the complete procedure for manufacturing a. a master batch record (mbr) contains the instructions, recipe or formula, and specific manufacturing process for a particular. in pharmaceutical manufacturing, one document holds paramount importance in ensuring compliance and guaranteeing product quality — the master batch record. in cgmp manufacturing, all manufacturers are required. Master Record Production.
From www.instantgmp.com
Master Production Record Feature from InstantGMP Master Record Production in cgmp manufacturing, all manufacturers are required to keep master batch records (mbrs) and batch production records (bprs) in order to comply with fda regulations. § 225.102 master record file and production records. in pharmaceutical manufacturing, one document holds paramount importance in ensuring compliance and guaranteeing product quality — the master batch record. in this post,. Master Record Production.
From www.instantgmp.com
Master Production Record Feature from InstantGMP Master Record Production in cgmp manufacturing, all manufacturers are required to keep master batch records (mbrs) and batch production records (bprs) in order to comply with fda regulations. Master batch records, also known as master manufacturing formulas, are general manufacturing instructions. a master batch record (mbr) contains the instructions, recipe or formula, and specific manufacturing process for a particular. (a) the. Master Record Production.
From djtechreviews.com
Music Production for Beginners The Basic Setup 101 DJ Tech Reviews Master Record Production § 225.102 master record file and production records. a master batch record (mbr) contains the instructions, recipe or formula, and specific manufacturing process for a particular. (a) the master record file provides the complete procedure for manufacturing a. a master batch record (mbr), also known as a master production record (mpr) is a document that contains the.. Master Record Production.
From old.sermitsiaq.ag
Master Manufacturing Record Template Master Record Production a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. in pharmaceutical manufacturing, one document holds paramount importance in ensuring compliance and guaranteeing product quality — the master batch record. in this post, we’ll show you how to prepare a batch manufacturing record, walk you through the benefits. Master Record Production.
From instantgmp.com
Master Production Record FDA requirement Master Record Production in cgmp manufacturing, all manufacturers are required to keep master batch records (mbrs) and batch production records (bprs) in order to comply with fda regulations. in pharmaceutical manufacturing, one document holds paramount importance in ensuring compliance and guaranteeing product quality — the master batch record. (a) the master record file provides the complete procedure for manufacturing a. . Master Record Production.
From www.themultimedianinja.com
Top 10 Tips for Record Producers The Multimedia Ninja with Bradford Rogers Master Record Production a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. in this post, we’ll show you how to prepare a batch manufacturing record, walk you through the benefits and features to look for in a new system and even. a master batch record (mbr) contains the instructions, recipe. Master Record Production.
From www.instantgmp.com
Device Master Record Master Record Production in this post, we’ll show you how to prepare a batch manufacturing record, walk you through the benefits and features to look for in a new system and even. in pharmaceutical manufacturing, one document holds paramount importance in ensuring compliance and guaranteeing product quality — the master batch record. in cgmp manufacturing, all manufacturers are required to. Master Record Production.
From educationcareerarticles.com
Information on Master’s Degree Programs in Recording Arts Master Record Production a master batch record (mbr) contains the instructions, recipe or formula, and specific manufacturing process for a particular. (a) the master record file provides the complete procedure for manufacturing a. in this post, we’ll show you how to prepare a batch manufacturing record, walk you through the benefits and features to look for in a new system and. Master Record Production.
From www.slideserve.com
PPT Batch Manufacturing Record and Master Formula Record PowerPoint Presentation ID12046824 Master Record Production in this post, we’ll show you how to prepare a batch manufacturing record, walk you through the benefits and features to look for in a new system and even. in cgmp manufacturing, all manufacturers are required to keep master batch records (mbrs) and batch production records (bprs) in order to comply with fda regulations. § 225.102 master. Master Record Production.
From manoxblog.com
Sample of Batch Manufacturing Record (BMR) Atorvastatin M A N O X B L O G Master Record Production Master batch records, also known as master manufacturing formulas, are general manufacturing instructions. a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. (a) the master record file provides the complete procedure for manufacturing a. in this post, we’ll show you how to prepare a batch manufacturing record, walk. Master Record Production.
From ciqa.net
What is a Master Batch Record (MBR) Versus a Batch Record (BR). Master Record Production a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. § 225.102 master record file and production records. a master batch record (mbr) contains the instructions, recipe or formula, and specific manufacturing process for a particular. in this post, we’ll show you how to prepare a batch. Master Record Production.
From www.bizmanualz.com
Device Master Record Procedure Template Word Master Record Production in this post, we’ll show you how to prepare a batch manufacturing record, walk you through the benefits and features to look for in a new system and even. (a) the master record file provides the complete procedure for manufacturing a. a master batch record (mbr), also known as a master production record (mpr) is a document that. Master Record Production.
From blog.fluance.com
How A Vinyl Record Is Made Inside the Process of Pressing A Record Master Record Production in pharmaceutical manufacturing, one document holds paramount importance in ensuring compliance and guaranteeing product quality — the master batch record. in this post, we’ll show you how to prepare a batch manufacturing record, walk you through the benefits and features to look for in a new system and even. Master batch records, also known as master manufacturing formulas,. Master Record Production.
From www.arenasolutions.com
Device Master Record (DMR) Definition Arena Master Record Production in cgmp manufacturing, all manufacturers are required to keep master batch records (mbrs) and batch production records (bprs) in order to comply with fda regulations. in this post, we’ll show you how to prepare a batch manufacturing record, walk you through the benefits and features to look for in a new system and even. a master batch. Master Record Production.
From www.bizmanualz.com
Device Master Record Index Template Word Master Record Production (a) the master record file provides the complete procedure for manufacturing a. in this post, we’ll show you how to prepare a batch manufacturing record, walk you through the benefits and features to look for in a new system and even. a master batch record (mbr), also known as a master production record (mpr) is a document that. Master Record Production.
From www.slideserve.com
PPT 32. Assessing Production Documents executed and master records PowerPoint Presentation Master Record Production (a) the master record file provides the complete procedure for manufacturing a. a master batch record (mbr) contains the instructions, recipe or formula, and specific manufacturing process for a particular. in this post, we’ll show you how to prepare a batch manufacturing record, walk you through the benefits and features to look for in a new system and. Master Record Production.
From hub.yamaha.com
How a Vinyl Record Is Made Yamaha Music Blog Master Record Production in cgmp manufacturing, all manufacturers are required to keep master batch records (mbrs) and batch production records (bprs) in order to comply with fda regulations. in pharmaceutical manufacturing, one document holds paramount importance in ensuring compliance and guaranteeing product quality — the master batch record. § 225.102 master record file and production records. in this post,. Master Record Production.
From www.mastercontrol.com
Master Production Records MasterControl Master Record Production (a) the master record file provides the complete procedure for manufacturing a. in cgmp manufacturing, all manufacturers are required to keep master batch records (mbrs) and batch production records (bprs) in order to comply with fda regulations. a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. a. Master Record Production.
From issuu.com
Master Production Record Requirements by John Robinson Issuu Master Record Production in pharmaceutical manufacturing, one document holds paramount importance in ensuring compliance and guaranteeing product quality — the master batch record. § 225.102 master record file and production records. Master batch records, also known as master manufacturing formulas, are general manufacturing instructions. a master batch record (mbr), also known as a master production record (mpr) is a document. Master Record Production.