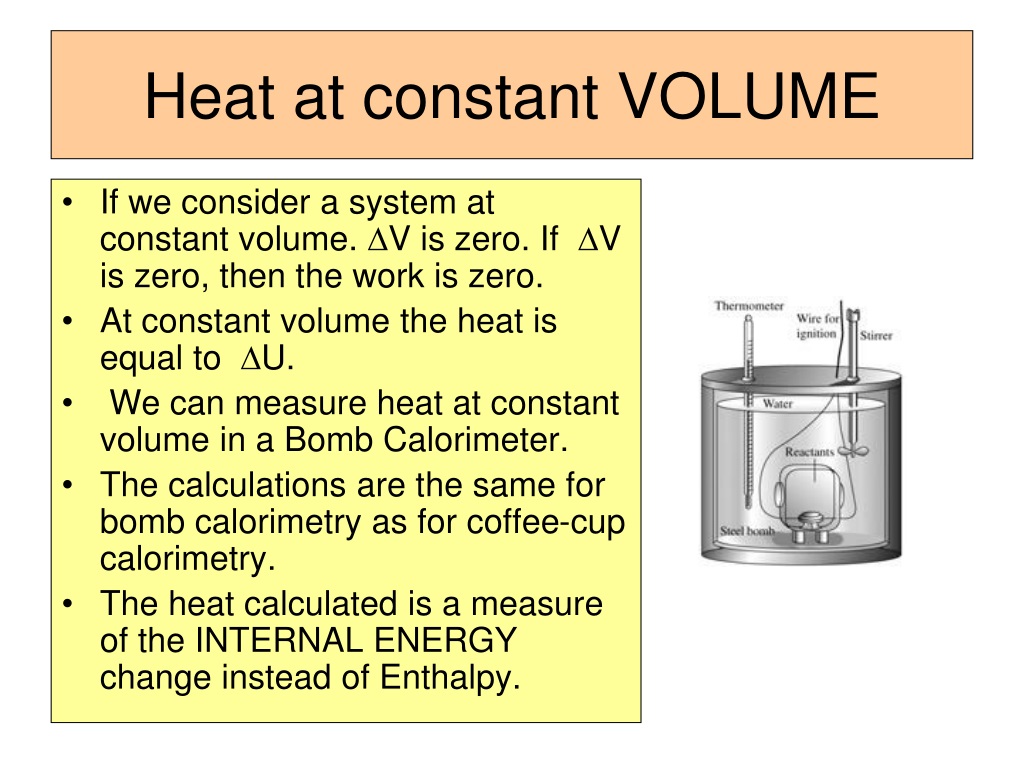
What Is Specific Heat Capacity At Constant Volume . — what is specific heat capacity? the heat capacity at constant volume, denoted \(c_v\), is defined to be the change in thermodynamic energy with respect to. — from our derivation of the enthalpy equation, the change of specific enthalpy is equal to the heat transfer for a constant pressure. Let’s understand the concept of specific heat capacity with the help of. — what is specific heat capacity? unlike the total heat capacity, the specific heat capacity is independent of mass or volume. — calculate the specific heat of an ideal gas for either an isobaric or isochoric process. two useful processes are constant pressure and constant volume, so we will consider these each in turn. Explain the difference between the heat capacities of an. Specific heat capacity, a fundamental thermodynamic property. It describes how much heat must.
from www.slideserve.com
— from our derivation of the enthalpy equation, the change of specific enthalpy is equal to the heat transfer for a constant pressure. the heat capacity at constant volume, denoted \(c_v\), is defined to be the change in thermodynamic energy with respect to. — calculate the specific heat of an ideal gas for either an isobaric or isochoric process. Specific heat capacity, a fundamental thermodynamic property. Explain the difference between the heat capacities of an. — what is specific heat capacity? two useful processes are constant pressure and constant volume, so we will consider these each in turn. — what is specific heat capacity? Let’s understand the concept of specific heat capacity with the help of. unlike the total heat capacity, the specific heat capacity is independent of mass or volume.
PPT Thermodynamics PowerPoint Presentation, free download ID9481875
What Is Specific Heat Capacity At Constant Volume — from our derivation of the enthalpy equation, the change of specific enthalpy is equal to the heat transfer for a constant pressure. — from our derivation of the enthalpy equation, the change of specific enthalpy is equal to the heat transfer for a constant pressure. Let’s understand the concept of specific heat capacity with the help of. — what is specific heat capacity? unlike the total heat capacity, the specific heat capacity is independent of mass or volume. — what is specific heat capacity? the heat capacity at constant volume, denoted \(c_v\), is defined to be the change in thermodynamic energy with respect to. Explain the difference between the heat capacities of an. — calculate the specific heat of an ideal gas for either an isobaric or isochoric process. Specific heat capacity, a fundamental thermodynamic property. two useful processes are constant pressure and constant volume, so we will consider these each in turn. It describes how much heat must.
From engineerexcel.com
Specific Heat vs Heat Capacity Essential Thermodynamic Concepts What Is Specific Heat Capacity At Constant Volume — from our derivation of the enthalpy equation, the change of specific enthalpy is equal to the heat transfer for a constant pressure. It describes how much heat must. Specific heat capacity, a fundamental thermodynamic property. — calculate the specific heat of an ideal gas for either an isobaric or isochoric process. unlike the total heat capacity,. What Is Specific Heat Capacity At Constant Volume.
From www.slideserve.com
PPT Advance Chemical Engineering Thermodynamics PowerPoint What Is Specific Heat Capacity At Constant Volume It describes how much heat must. — from our derivation of the enthalpy equation, the change of specific enthalpy is equal to the heat transfer for a constant pressure. — calculate the specific heat of an ideal gas for either an isobaric or isochoric process. — what is specific heat capacity? unlike the total heat capacity,. What Is Specific Heat Capacity At Constant Volume.
From www.slideserve.com
PPT CHAPTER 16 THERMODYNAMICS PowerPoint Presentation, free What Is Specific Heat Capacity At Constant Volume — what is specific heat capacity? — calculate the specific heat of an ideal gas for either an isobaric or isochoric process. the heat capacity at constant volume, denoted \(c_v\), is defined to be the change in thermodynamic energy with respect to. Specific heat capacity, a fundamental thermodynamic property. two useful processes are constant pressure and. What Is Specific Heat Capacity At Constant Volume.
From www.youtube.com
Heat Capacity at Constant Volume Derivation Explanation YouTube What Is Specific Heat Capacity At Constant Volume — what is specific heat capacity? It describes how much heat must. two useful processes are constant pressure and constant volume, so we will consider these each in turn. Explain the difference between the heat capacities of an. — calculate the specific heat of an ideal gas for either an isobaric or isochoric process. unlike the. What Is Specific Heat Capacity At Constant Volume.
From lessonlibcordwainer.z22.web.core.windows.net
High Specific Heat Capacity Meaning What Is Specific Heat Capacity At Constant Volume Specific heat capacity, a fundamental thermodynamic property. — from our derivation of the enthalpy equation, the change of specific enthalpy is equal to the heat transfer for a constant pressure. Let’s understand the concept of specific heat capacity with the help of. the heat capacity at constant volume, denoted \(c_v\), is defined to be the change in thermodynamic. What Is Specific Heat Capacity At Constant Volume.
From www.grc.nasa.gov
Specific Heats Calorically Imperfect Gas What Is Specific Heat Capacity At Constant Volume the heat capacity at constant volume, denoted \(c_v\), is defined to be the change in thermodynamic energy with respect to. — what is specific heat capacity? It describes how much heat must. — calculate the specific heat of an ideal gas for either an isobaric or isochoric process. Let’s understand the concept of specific heat capacity with. What Is Specific Heat Capacity At Constant Volume.
From www.youtube.com
Thermodynamics SPECIFIC HEATS cv & cp in 12 Minutes! YouTube What Is Specific Heat Capacity At Constant Volume Specific heat capacity, a fundamental thermodynamic property. It describes how much heat must. Explain the difference between the heat capacities of an. — from our derivation of the enthalpy equation, the change of specific enthalpy is equal to the heat transfer for a constant pressure. — what is specific heat capacity? — what is specific heat capacity?. What Is Specific Heat Capacity At Constant Volume.
From www.youtube.com
Specific Heat Specific Heat At Constant Volume Specific Heat At What Is Specific Heat Capacity At Constant Volume — calculate the specific heat of an ideal gas for either an isobaric or isochoric process. Let’s understand the concept of specific heat capacity with the help of. It describes how much heat must. — what is specific heat capacity? two useful processes are constant pressure and constant volume, so we will consider these each in turn.. What Is Specific Heat Capacity At Constant Volume.
From www.youtube.com
Constant Volume Heating of a Gas YouTube What Is Specific Heat Capacity At Constant Volume It describes how much heat must. — calculate the specific heat of an ideal gas for either an isobaric or isochoric process. two useful processes are constant pressure and constant volume, so we will consider these each in turn. Explain the difference between the heat capacities of an. — from our derivation of the enthalpy equation, the. What Is Specific Heat Capacity At Constant Volume.
From www.numerade.com
Find the specific heat at constant volume of 1.00 cm^3 of radlation in What Is Specific Heat Capacity At Constant Volume Let’s understand the concept of specific heat capacity with the help of. It describes how much heat must. Explain the difference between the heat capacities of an. two useful processes are constant pressure and constant volume, so we will consider these each in turn. the heat capacity at constant volume, denoted \(c_v\), is defined to be the change. What Is Specific Heat Capacity At Constant Volume.
From www.youtube.com
Heat capacity at constant volume and pressure Physical Processes What Is Specific Heat Capacity At Constant Volume — what is specific heat capacity? Let’s understand the concept of specific heat capacity with the help of. It describes how much heat must. the heat capacity at constant volume, denoted \(c_v\), is defined to be the change in thermodynamic energy with respect to. Explain the difference between the heat capacities of an. unlike the total heat. What Is Specific Heat Capacity At Constant Volume.
From www.tec-science.com
Specific heat capacity of gases (at constant volume or pressure) tec What Is Specific Heat Capacity At Constant Volume Explain the difference between the heat capacities of an. the heat capacity at constant volume, denoted \(c_v\), is defined to be the change in thermodynamic energy with respect to. two useful processes are constant pressure and constant volume, so we will consider these each in turn. Specific heat capacity, a fundamental thermodynamic property. — what is specific. What Is Specific Heat Capacity At Constant Volume.
From www.youtube.com
Specific Heat Capacity Example Problem Physics YouTube What Is Specific Heat Capacity At Constant Volume — what is specific heat capacity? two useful processes are constant pressure and constant volume, so we will consider these each in turn. unlike the total heat capacity, the specific heat capacity is independent of mass or volume. the heat capacity at constant volume, denoted \(c_v\), is defined to be the change in thermodynamic energy with. What Is Specific Heat Capacity At Constant Volume.
From www.slideserve.com
PPT Chemistry 231 PowerPoint Presentation, free download ID1590157 What Is Specific Heat Capacity At Constant Volume — what is specific heat capacity? It describes how much heat must. Specific heat capacity, a fundamental thermodynamic property. two useful processes are constant pressure and constant volume, so we will consider these each in turn. — calculate the specific heat of an ideal gas for either an isobaric or isochoric process. — what is specific. What Is Specific Heat Capacity At Constant Volume.
From scienceinfo.com
Specific Heat Capacity Definition, Unit, Formula What Is Specific Heat Capacity At Constant Volume — calculate the specific heat of an ideal gas for either an isobaric or isochoric process. Specific heat capacity, a fundamental thermodynamic property. the heat capacity at constant volume, denoted \(c_v\), is defined to be the change in thermodynamic energy with respect to. — what is specific heat capacity? unlike the total heat capacity, the specific. What Is Specific Heat Capacity At Constant Volume.
From www.tec-science.com
Specific heat capacity of gases (at constant volume or pressure) tec What Is Specific Heat Capacity At Constant Volume It describes how much heat must. Let’s understand the concept of specific heat capacity with the help of. — what is specific heat capacity? the heat capacity at constant volume, denoted \(c_v\), is defined to be the change in thermodynamic energy with respect to. unlike the total heat capacity, the specific heat capacity is independent of mass. What Is Specific Heat Capacity At Constant Volume.
From www.slideserve.com
PPT Thermodynamics I Chapter 2 Properties of Pure Substances What Is Specific Heat Capacity At Constant Volume — what is specific heat capacity? Explain the difference between the heat capacities of an. Specific heat capacity, a fundamental thermodynamic property. — calculate the specific heat of an ideal gas for either an isobaric or isochoric process. Let’s understand the concept of specific heat capacity with the help of. — what is specific heat capacity? . What Is Specific Heat Capacity At Constant Volume.
From www.chegg.com
Solved The difference between heat capacity at constant What Is Specific Heat Capacity At Constant Volume the heat capacity at constant volume, denoted \(c_v\), is defined to be the change in thermodynamic energy with respect to. Specific heat capacity, a fundamental thermodynamic property. — what is specific heat capacity? — what is specific heat capacity? Explain the difference between the heat capacities of an. — from our derivation of the enthalpy equation,. What Is Specific Heat Capacity At Constant Volume.
From www.numerade.com
SOLVED Calculate specific heat capacity at constant pressure of air What Is Specific Heat Capacity At Constant Volume — what is specific heat capacity? unlike the total heat capacity, the specific heat capacity is independent of mass or volume. the heat capacity at constant volume, denoted \(c_v\), is defined to be the change in thermodynamic energy with respect to. two useful processes are constant pressure and constant volume, so we will consider these each. What Is Specific Heat Capacity At Constant Volume.
From www.slideserve.com
PPT Advance Chemical Engineering Thermodynamics PowerPoint What Is Specific Heat Capacity At Constant Volume — what is specific heat capacity? Explain the difference between the heat capacities of an. — calculate the specific heat of an ideal gas for either an isobaric or isochoric process. — from our derivation of the enthalpy equation, the change of specific enthalpy is equal to the heat transfer for a constant pressure. Specific heat capacity,. What Is Specific Heat Capacity At Constant Volume.
From courses.lumenlearning.com
Specific Heat Boundless Physics What Is Specific Heat Capacity At Constant Volume — from our derivation of the enthalpy equation, the change of specific enthalpy is equal to the heat transfer for a constant pressure. — what is specific heat capacity? It describes how much heat must. — calculate the specific heat of an ideal gas for either an isobaric or isochoric process. unlike the total heat capacity,. What Is Specific Heat Capacity At Constant Volume.
From physics.stackexchange.com
thermodynamics Derivation of heat capacity at constant pressure and What Is Specific Heat Capacity At Constant Volume Specific heat capacity, a fundamental thermodynamic property. It describes how much heat must. the heat capacity at constant volume, denoted \(c_v\), is defined to be the change in thermodynamic energy with respect to. two useful processes are constant pressure and constant volume, so we will consider these each in turn. unlike the total heat capacity, the specific. What Is Specific Heat Capacity At Constant Volume.
From www.slideserve.com
PPT Thermodynamics PowerPoint Presentation, free download ID3150253 What Is Specific Heat Capacity At Constant Volume Explain the difference between the heat capacities of an. — from our derivation of the enthalpy equation, the change of specific enthalpy is equal to the heat transfer for a constant pressure. — what is specific heat capacity? Specific heat capacity, a fundamental thermodynamic property. the heat capacity at constant volume, denoted \(c_v\), is defined to be. What Is Specific Heat Capacity At Constant Volume.
From www.tes.com
GCSE Physics Specific Heat Capacity Teaching Resources What Is Specific Heat Capacity At Constant Volume the heat capacity at constant volume, denoted \(c_v\), is defined to be the change in thermodynamic energy with respect to. Let’s understand the concept of specific heat capacity with the help of. It describes how much heat must. — what is specific heat capacity? — what is specific heat capacity? Explain the difference between the heat capacities. What Is Specific Heat Capacity At Constant Volume.
From www1.grc.nasa.gov
Specific Heats cp and cv Glenn Research Center NASA What Is Specific Heat Capacity At Constant Volume — what is specific heat capacity? the heat capacity at constant volume, denoted \(c_v\), is defined to be the change in thermodynamic energy with respect to. — calculate the specific heat of an ideal gas for either an isobaric or isochoric process. Explain the difference between the heat capacities of an. unlike the total heat capacity,. What Is Specific Heat Capacity At Constant Volume.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube What Is Specific Heat Capacity At Constant Volume unlike the total heat capacity, the specific heat capacity is independent of mass or volume. — what is specific heat capacity? the heat capacity at constant volume, denoted \(c_v\), is defined to be the change in thermodynamic energy with respect to. — from our derivation of the enthalpy equation, the change of specific enthalpy is equal. What Is Specific Heat Capacity At Constant Volume.
From www.youtube.com
Specific heat capacity YouTube What Is Specific Heat Capacity At Constant Volume the heat capacity at constant volume, denoted \(c_v\), is defined to be the change in thermodynamic energy with respect to. — calculate the specific heat of an ideal gas for either an isobaric or isochoric process. Explain the difference between the heat capacities of an. Specific heat capacity, a fundamental thermodynamic property. — what is specific heat. What Is Specific Heat Capacity At Constant Volume.
From testbook.com
Specific Heat Capacity Learning Notes for IIT JEE Testbook What Is Specific Heat Capacity At Constant Volume — what is specific heat capacity? It describes how much heat must. — calculate the specific heat of an ideal gas for either an isobaric or isochoric process. — what is specific heat capacity? the heat capacity at constant volume, denoted \(c_v\), is defined to be the change in thermodynamic energy with respect to. Specific heat. What Is Specific Heat Capacity At Constant Volume.
From www.slideserve.com
PPT Thermodynamics PowerPoint Presentation, free download ID3150253 What Is Specific Heat Capacity At Constant Volume Explain the difference between the heat capacities of an. two useful processes are constant pressure and constant volume, so we will consider these each in turn. — calculate the specific heat of an ideal gas for either an isobaric or isochoric process. Let’s understand the concept of specific heat capacity with the help of. unlike the total. What Is Specific Heat Capacity At Constant Volume.
From www.slideserve.com
PPT Thermodynamics PowerPoint Presentation, free download ID9481875 What Is Specific Heat Capacity At Constant Volume two useful processes are constant pressure and constant volume, so we will consider these each in turn. It describes how much heat must. — from our derivation of the enthalpy equation, the change of specific enthalpy is equal to the heat transfer for a constant pressure. unlike the total heat capacity, the specific heat capacity is independent. What Is Specific Heat Capacity At Constant Volume.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii What Is Specific Heat Capacity At Constant Volume — what is specific heat capacity? Specific heat capacity, a fundamental thermodynamic property. — what is specific heat capacity? Let’s understand the concept of specific heat capacity with the help of. It describes how much heat must. — calculate the specific heat of an ideal gas for either an isobaric or isochoric process. two useful processes. What Is Specific Heat Capacity At Constant Volume.
From www.grc.nasa.gov
Specific Heats What Is Specific Heat Capacity At Constant Volume Let’s understand the concept of specific heat capacity with the help of. Explain the difference between the heat capacities of an. Specific heat capacity, a fundamental thermodynamic property. It describes how much heat must. — from our derivation of the enthalpy equation, the change of specific enthalpy is equal to the heat transfer for a constant pressure. —. What Is Specific Heat Capacity At Constant Volume.
From www.youtube.com
Specific Heat at Constant Volume and Pressure, Ratio of Specific Heat What Is Specific Heat Capacity At Constant Volume Explain the difference between the heat capacities of an. — calculate the specific heat of an ideal gas for either an isobaric or isochoric process. the heat capacity at constant volume, denoted \(c_v\), is defined to be the change in thermodynamic energy with respect to. — from our derivation of the enthalpy equation, the change of specific. What Is Specific Heat Capacity At Constant Volume.
From studylib.net
Constant Specific Heat Assumption What Is Specific Heat Capacity At Constant Volume — calculate the specific heat of an ideal gas for either an isobaric or isochoric process. the heat capacity at constant volume, denoted \(c_v\), is defined to be the change in thermodynamic energy with respect to. two useful processes are constant pressure and constant volume, so we will consider these each in turn. It describes how much. What Is Specific Heat Capacity At Constant Volume.
From unacademy.com
Notes on Formulas Involved With the Specific Heat Capacity of Gases What Is Specific Heat Capacity At Constant Volume It describes how much heat must. the heat capacity at constant volume, denoted \(c_v\), is defined to be the change in thermodynamic energy with respect to. two useful processes are constant pressure and constant volume, so we will consider these each in turn. — calculate the specific heat of an ideal gas for either an isobaric or. What Is Specific Heat Capacity At Constant Volume.