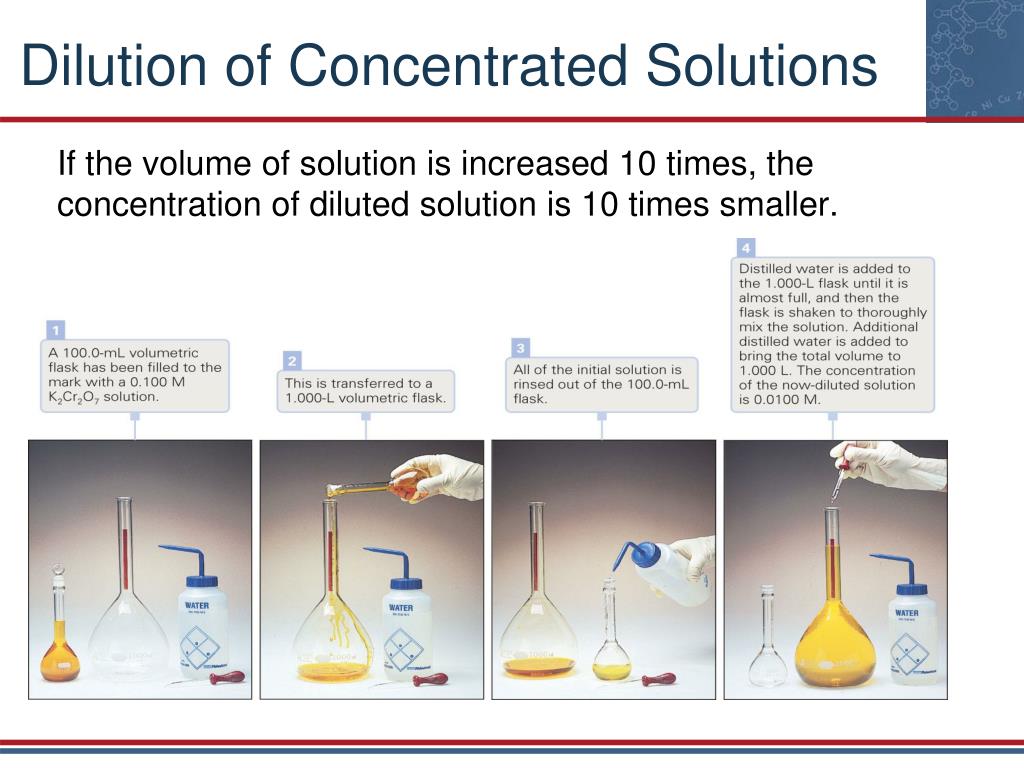
Dilute Solution Characteristics . Dilute solutions have a lower density and lighter color compared to concentrated solutions, while unsaturated solutions have the ability to dissolve. Calculate the molarity of a solution. In such solutions, the concentration of solute is. Dilution is the process of weakening or deconcentrating a solution. A solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). A common method of making a solution of a given concentration involves taking a more concentration solution and adding water until the desired. A dilute solution is a mixture where a small amount of solute is present relative to the solvent. A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of solvent (the. Serial dilutions are used in microbiology to reduce bacterial concentrations. Use the terms concentrated and dilute to describe the relative concentration of a solution. A volumetric flask is usually used to prepare a stock solution.
from www.slideserve.com
Dilution is the process of weakening or deconcentrating a solution. Dilute solutions have a lower density and lighter color compared to concentrated solutions, while unsaturated solutions have the ability to dissolve. A common method of making a solution of a given concentration involves taking a more concentration solution and adding water until the desired. Serial dilutions are used in microbiology to reduce bacterial concentrations. Calculate the molarity of a solution. A solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of solvent (the. In such solutions, the concentration of solute is. A volumetric flask is usually used to prepare a stock solution. Use the terms concentrated and dilute to describe the relative concentration of a solution.
PPT Chapter 16 Solutions PowerPoint Presentation, free download ID
Dilute Solution Characteristics A dilute solution is a mixture where a small amount of solute is present relative to the solvent. A common method of making a solution of a given concentration involves taking a more concentration solution and adding water until the desired. A solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). Calculate the molarity of a solution. Dilute solutions have a lower density and lighter color compared to concentrated solutions, while unsaturated solutions have the ability to dissolve. Use the terms concentrated and dilute to describe the relative concentration of a solution. Dilution is the process of weakening or deconcentrating a solution. A volumetric flask is usually used to prepare a stock solution. A dilute solution is a mixture where a small amount of solute is present relative to the solvent. In such solutions, the concentration of solute is. A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of solvent (the. Serial dilutions are used in microbiology to reduce bacterial concentrations.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID5812279 Dilute Solution Characteristics Serial dilutions are used in microbiology to reduce bacterial concentrations. Calculate the molarity of a solution. A dilute solution is a mixture where a small amount of solute is present relative to the solvent. A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of solvent (the.. Dilute Solution Characteristics.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Dilute Solution Characteristics A volumetric flask is usually used to prepare a stock solution. A solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). Dilute solutions have a lower density and lighter color compared to concentrated solutions, while unsaturated solutions have the ability to dissolve. Dilution is the process of weakening. Dilute Solution Characteristics.
From fity.club
Dilute Definition Dilute Solution Characteristics In such solutions, the concentration of solute is. A dilute solution is a mixture where a small amount of solute is present relative to the solvent. Calculate the molarity of a solution. Use the terms concentrated and dilute to describe the relative concentration of a solution. Dilution is the process of weakening or deconcentrating a solution. A solution containing a. Dilute Solution Characteristics.
From www.youtube.com
What is Dilute Solution? Chemistry YouTube Dilute Solution Characteristics A common method of making a solution of a given concentration involves taking a more concentration solution and adding water until the desired. Serial dilutions are used in microbiology to reduce bacterial concentrations. In such solutions, the concentration of solute is. Dilute solutions have a lower density and lighter color compared to concentrated solutions, while unsaturated solutions have the ability. Dilute Solution Characteristics.
From www.slideserve.com
PPT Grade 7 Science PowerPoint Presentation, free download ID2229802 Dilute Solution Characteristics In such solutions, the concentration of solute is. A dilute solution is a mixture where a small amount of solute is present relative to the solvent. A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of solvent (the. A volumetric flask is usually used to prepare. Dilute Solution Characteristics.
From klafwpnrs.blob.core.windows.net
Dilution Formula Explanation at John Schell blog Dilute Solution Characteristics A dilute solution is a mixture where a small amount of solute is present relative to the solvent. Calculate the molarity of a solution. Dilution is the process of weakening or deconcentrating a solution. A common method of making a solution of a given concentration involves taking a more concentration solution and adding water until the desired. Use the terms. Dilute Solution Characteristics.
From klakbhtvf.blob.core.windows.net
Dilute Solution Has What at Virginia Sebastian blog Dilute Solution Characteristics A dilute solution is a mixture where a small amount of solute is present relative to the solvent. Dilute solutions have a lower density and lighter color compared to concentrated solutions, while unsaturated solutions have the ability to dissolve. In such solutions, the concentration of solute is. A dilute solution is one in which the amount of solute (the substance. Dilute Solution Characteristics.
From laxenian.weebly.com
How To Dilute A Solution laxenian Dilute Solution Characteristics A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of solvent (the. Dilute solutions have a lower density and lighter color compared to concentrated solutions, while unsaturated solutions have the ability to dissolve. Dilution is the process of weakening or deconcentrating a solution. Calculate the molarity. Dilute Solution Characteristics.
From www.askiitians.com
Types Of Solutions Study Material for IIT JEE askIITians Dilute Solution Characteristics A dilute solution is a mixture where a small amount of solute is present relative to the solvent. In such solutions, the concentration of solute is. Use the terms concentrated and dilute to describe the relative concentration of a solution. A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared. Dilute Solution Characteristics.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download Dilute Solution Characteristics A dilute solution is a mixture where a small amount of solute is present relative to the solvent. A common method of making a solution of a given concentration involves taking a more concentration solution and adding water until the desired. A solution containing a precise mass of solute in a precise volume of solution is called a stock solution. Dilute Solution Characteristics.
From www.youtube.com
pH of Dilute Solution (Example) YouTube Dilute Solution Characteristics A common method of making a solution of a given concentration involves taking a more concentration solution and adding water until the desired. A dilute solution is a mixture where a small amount of solute is present relative to the solvent. Use the terms concentrated and dilute to describe the relative concentration of a solution. Calculate the molarity of a. Dilute Solution Characteristics.
From slideplayer.com
Solutions Chapter ppt download Dilute Solution Characteristics A volumetric flask is usually used to prepare a stock solution. A dilute solution is a mixture where a small amount of solute is present relative to the solvent. A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of solvent (the. A solution containing a precise. Dilute Solution Characteristics.
From giotapowu.blob.core.windows.net
How Does A Dilute Solution Work at Annie Ahmed blog Dilute Solution Characteristics A volumetric flask is usually used to prepare a stock solution. Serial dilutions are used in microbiology to reduce bacterial concentrations. In such solutions, the concentration of solute is. Calculate the molarity of a solution. A solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). A dilute solution. Dilute Solution Characteristics.
From www.istockphoto.com
Concentrated Solution Vs Dilute Solution Stock Illustration Download Dilute Solution Characteristics A volumetric flask is usually used to prepare a stock solution. In such solutions, the concentration of solute is. Calculate the molarity of a solution. Use the terms concentrated and dilute to describe the relative concentration of a solution. A solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard. Dilute Solution Characteristics.
From www.youtube.com
Dilute, Concentrated and saturated solution YouTube Dilute Solution Characteristics A common method of making a solution of a given concentration involves taking a more concentration solution and adding water until the desired. Dilution is the process of weakening or deconcentrating a solution. A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of solvent (the. A. Dilute Solution Characteristics.
From ar.inspiredpencil.com
Dilute Science Dilute Solution Characteristics In such solutions, the concentration of solute is. Use the terms concentrated and dilute to describe the relative concentration of a solution. A common method of making a solution of a given concentration involves taking a more concentration solution and adding water until the desired. Serial dilutions are used in microbiology to reduce bacterial concentrations. Calculate the molarity of a. Dilute Solution Characteristics.
From www.slideserve.com
PPT Chapter 15 Solutions PowerPoint Presentation, free download ID Dilute Solution Characteristics A common method of making a solution of a given concentration involves taking a more concentration solution and adding water until the desired. In such solutions, the concentration of solute is. Calculate the molarity of a solution. A volumetric flask is usually used to prepare a stock solution. A solution containing a precise mass of solute in a precise volume. Dilute Solution Characteristics.
From slideplayer.com
Chapter 22 Solutions Lesson ppt download Dilute Solution Characteristics In such solutions, the concentration of solute is. Serial dilutions are used in microbiology to reduce bacterial concentrations. Dilution is the process of weakening or deconcentrating a solution. Calculate the molarity of a solution. Use the terms concentrated and dilute to describe the relative concentration of a solution. Dilute solutions have a lower density and lighter color compared to concentrated. Dilute Solution Characteristics.
From www.youtube.com
TYPES OF SOLUTION,Dilution of Solution,Concentrated,Dilute solutions Dilute Solution Characteristics Dilute solutions have a lower density and lighter color compared to concentrated solutions, while unsaturated solutions have the ability to dissolve. A common method of making a solution of a given concentration involves taking a more concentration solution and adding water until the desired. Use the terms concentrated and dilute to describe the relative concentration of a solution. In such. Dilute Solution Characteristics.
From www.pdfprof.com
how to dilute solutions Dilute Solution Characteristics A dilute solution is a mixture where a small amount of solute is present relative to the solvent. A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of solvent (the. In such solutions, the concentration of solute is. A solution containing a precise mass of solute. Dilute Solution Characteristics.
From www.pinterest.com
Dilute Flashcards, Easy science, Science student Dilute Solution Characteristics A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of solvent (the. A dilute solution is a mixture where a small amount of solute is present relative to the solvent. A volumetric flask is usually used to prepare a stock solution. Calculate the molarity of a. Dilute Solution Characteristics.
From ar.inspiredpencil.com
Dilute Solution Diagram Dilute Solution Characteristics A solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). A volumetric flask is usually used to prepare a stock solution. Serial dilutions are used in microbiology to reduce bacterial concentrations. Dilution is the process of weakening or deconcentrating a solution. In such solutions, the concentration of solute. Dilute Solution Characteristics.
From klaivqdcu.blob.core.windows.net
What Does Dilute In Chemistry Mean at Sharron Wallace blog Dilute Solution Characteristics Dilution is the process of weakening or deconcentrating a solution. In such solutions, the concentration of solute is. Use the terms concentrated and dilute to describe the relative concentration of a solution. A solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). A volumetric flask is usually used. Dilute Solution Characteristics.
From exodffmqo.blob.core.windows.net
Dilute Salt Solutions at Thomas Roosevelt blog Dilute Solution Characteristics Dilution is the process of weakening or deconcentrating a solution. Serial dilutions are used in microbiology to reduce bacterial concentrations. Calculate the molarity of a solution. Dilute solutions have a lower density and lighter color compared to concentrated solutions, while unsaturated solutions have the ability to dissolve. A solution containing a precise mass of solute in a precise volume of. Dilute Solution Characteristics.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Dilute Solution Characteristics A volumetric flask is usually used to prepare a stock solution. A dilute solution is a mixture where a small amount of solute is present relative to the solvent. Serial dilutions are used in microbiology to reduce bacterial concentrations. In such solutions, the concentration of solute is. A common method of making a solution of a given concentration involves taking. Dilute Solution Characteristics.
From klaivqdcu.blob.core.windows.net
What Does Dilute In Chemistry Mean at Sharron Wallace blog Dilute Solution Characteristics A volumetric flask is usually used to prepare a stock solution. A dilute solution is a mixture where a small amount of solute is present relative to the solvent. Dilution is the process of weakening or deconcentrating a solution. Serial dilutions are used in microbiology to reduce bacterial concentrations. In such solutions, the concentration of solute is. Dilute solutions have. Dilute Solution Characteristics.
From www.slideserve.com
PPT Solutions Part II PowerPoint Presentation, free download ID4272422 Dilute Solution Characteristics A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of solvent (the. Serial dilutions are used in microbiology to reduce bacterial concentrations. A common method of making a solution of a given concentration involves taking a more concentration solution and adding water until the desired. A. Dilute Solution Characteristics.
From www.geeksforgeeks.org
Concentration of Solution Definition, Formulas & Solved Examples Dilute Solution Characteristics Calculate the molarity of a solution. A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of solvent (the. A volumetric flask is usually used to prepare a stock solution. Dilution is the process of weakening or deconcentrating a solution. Dilute solutions have a lower density and. Dilute Solution Characteristics.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilute Solution Characteristics A volumetric flask is usually used to prepare a stock solution. Dilution is the process of weakening or deconcentrating a solution. Use the terms concentrated and dilute to describe the relative concentration of a solution. Serial dilutions are used in microbiology to reduce bacterial concentrations. In such solutions, the concentration of solute is. A dilute solution is one in which. Dilute Solution Characteristics.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Dilute Solution Characteristics In such solutions, the concentration of solute is. A common method of making a solution of a given concentration involves taking a more concentration solution and adding water until the desired. Use the terms concentrated and dilute to describe the relative concentration of a solution. Serial dilutions are used in microbiology to reduce bacterial concentrations. Dilution is the process of. Dilute Solution Characteristics.
From klakbhtvf.blob.core.windows.net
Dilute Solution Has What at Virginia Sebastian blog Dilute Solution Characteristics Dilution is the process of weakening or deconcentrating a solution. A volumetric flask is usually used to prepare a stock solution. A dilute solution is a mixture where a small amount of solute is present relative to the solvent. Use the terms concentrated and dilute to describe the relative concentration of a solution. Dilute solutions have a lower density and. Dilute Solution Characteristics.
From www.researchgate.net
Characteristic concentration ranges from dilute solutions to molten Dilute Solution Characteristics A volumetric flask is usually used to prepare a stock solution. Use the terms concentrated and dilute to describe the relative concentration of a solution. A dilute solution is a mixture where a small amount of solute is present relative to the solvent. Dilution is the process of weakening or deconcentrating a solution. A solution containing a precise mass of. Dilute Solution Characteristics.
From slideplayer.com
Solutions. ppt download Dilute Solution Characteristics In such solutions, the concentration of solute is. Calculate the molarity of a solution. A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of solvent (the. A dilute solution is a mixture where a small amount of solute is present relative to the solvent. A solution. Dilute Solution Characteristics.
From www.coursehero.com
[Solved] . Model 2 Chemical Solutions Dilute Solution Concentrated Dilute Solution Characteristics A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of solvent (the. Calculate the molarity of a solution. A dilute solution is a mixture where a small amount of solute is present relative to the solvent. Dilute solutions have a lower density and lighter color compared. Dilute Solution Characteristics.
From www.slideserve.com
PPT Chapter 16 Solutions PowerPoint Presentation, free download ID Dilute Solution Characteristics A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of solvent (the. A volumetric flask is usually used to prepare a stock solution. In such solutions, the concentration of solute is. Use the terms concentrated and dilute to describe the relative concentration of a solution. Calculate. Dilute Solution Characteristics.