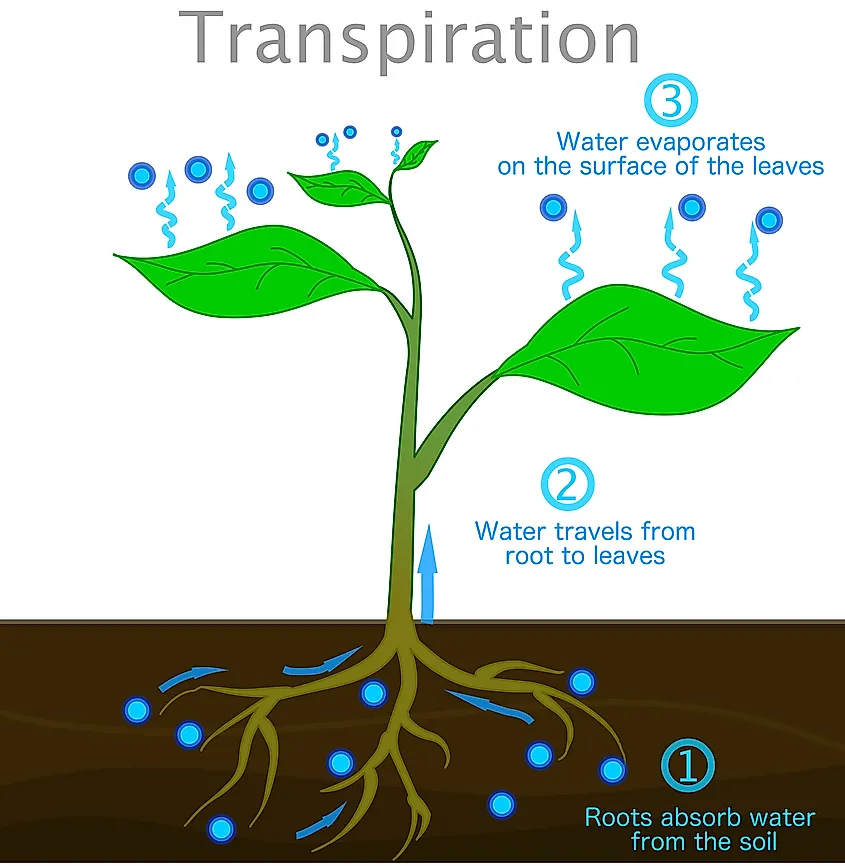
What Absorbs Water Molecules . As a result of water’s polarity, each water molecule attracts other water molecules because of the opposite charges between water molecules, forming hydrogen bonds. As a result of water’s polarity, each water molecule attracts other water molecules because of the opposite charges between water molecules, forming hydrogen bonds. Besides mercury, water has the highest surface tension for all liquids. Water also attracts or is attracted to other polar molecules and ions. When water vapor is absorbed, the water molecules are taken into the molecules of the hygroscopic substance, often resulting in physical changes, such as increased volume. They grow between soil particles and absorb water and minerals from the soil. Water's high surface tension is due to the hydrogen bonding in water. Water is absorbed by root hair cells in plants through osmosis which allows the plant to retain moisture that is needed to survive. Water enters the root hair cells by osmosis.
from www.worldatlas.com
When water vapor is absorbed, the water molecules are taken into the molecules of the hygroscopic substance, often resulting in physical changes, such as increased volume. As a result of water’s polarity, each water molecule attracts other water molecules because of the opposite charges between water molecules, forming hydrogen bonds. They grow between soil particles and absorb water and minerals from the soil. Water enters the root hair cells by osmosis. As a result of water’s polarity, each water molecule attracts other water molecules because of the opposite charges between water molecules, forming hydrogen bonds. Water is absorbed by root hair cells in plants through osmosis which allows the plant to retain moisture that is needed to survive. Water's high surface tension is due to the hydrogen bonding in water. Besides mercury, water has the highest surface tension for all liquids. Water also attracts or is attracted to other polar molecules and ions.
The Water Cycle WorldAtlas
What Absorbs Water Molecules Water's high surface tension is due to the hydrogen bonding in water. They grow between soil particles and absorb water and minerals from the soil. Water's high surface tension is due to the hydrogen bonding in water. As a result of water’s polarity, each water molecule attracts other water molecules because of the opposite charges between water molecules, forming hydrogen bonds. As a result of water’s polarity, each water molecule attracts other water molecules because of the opposite charges between water molecules, forming hydrogen bonds. Water also attracts or is attracted to other polar molecules and ions. Water is absorbed by root hair cells in plants through osmosis which allows the plant to retain moisture that is needed to survive. Water enters the root hair cells by osmosis. When water vapor is absorbed, the water molecules are taken into the molecules of the hygroscopic substance, often resulting in physical changes, such as increased volume. Besides mercury, water has the highest surface tension for all liquids.
From www.expii.com
Polar vs. Nonpolar Bonds — Overview & Examples Expii What Absorbs Water Molecules Water enters the root hair cells by osmosis. As a result of water’s polarity, each water molecule attracts other water molecules because of the opposite charges between water molecules, forming hydrogen bonds. They grow between soil particles and absorb water and minerals from the soil. When water vapor is absorbed, the water molecules are taken into the molecules of the. What Absorbs Water Molecules.
From sciencenotes.org
Adsorption vs Absorption Differences and Examples What Absorbs Water Molecules As a result of water’s polarity, each water molecule attracts other water molecules because of the opposite charges between water molecules, forming hydrogen bonds. Water is absorbed by root hair cells in plants through osmosis which allows the plant to retain moisture that is needed to survive. Water enters the root hair cells by osmosis. Water also attracts or is. What Absorbs Water Molecules.
From joieijlac.blob.core.windows.net
Water Definition Science at Corey Perez blog What Absorbs Water Molecules Water also attracts or is attracted to other polar molecules and ions. Water is absorbed by root hair cells in plants through osmosis which allows the plant to retain moisture that is needed to survive. They grow between soil particles and absorb water and minerals from the soil. Water enters the root hair cells by osmosis. When water vapor is. What Absorbs Water Molecules.
From exoudfseb.blob.core.windows.net
How Does Plants Absorb Water at Bradly Jamison blog What Absorbs Water Molecules Water enters the root hair cells by osmosis. Besides mercury, water has the highest surface tension for all liquids. Water also attracts or is attracted to other polar molecules and ions. When water vapor is absorbed, the water molecules are taken into the molecules of the hygroscopic substance, often resulting in physical changes, such as increased volume. As a result. What Absorbs Water Molecules.
From www.snexplores.org
Explainer What are the different states of matter? What Absorbs Water Molecules Water enters the root hair cells by osmosis. Besides mercury, water has the highest surface tension for all liquids. They grow between soil particles and absorb water and minerals from the soil. When water vapor is absorbed, the water molecules are taken into the molecules of the hygroscopic substance, often resulting in physical changes, such as increased volume. As a. What Absorbs Water Molecules.
From exoudfseb.blob.core.windows.net
How Does Plants Absorb Water at Bradly Jamison blog What Absorbs Water Molecules They grow between soil particles and absorb water and minerals from the soil. As a result of water’s polarity, each water molecule attracts other water molecules because of the opposite charges between water molecules, forming hydrogen bonds. Water's high surface tension is due to the hydrogen bonding in water. When water vapor is absorbed, the water molecules are taken into. What Absorbs Water Molecules.
From www.scienceabc.com
Is Water Polar Or Nonpolar? » ScienceABC What Absorbs Water Molecules As a result of water’s polarity, each water molecule attracts other water molecules because of the opposite charges between water molecules, forming hydrogen bonds. When water vapor is absorbed, the water molecules are taken into the molecules of the hygroscopic substance, often resulting in physical changes, such as increased volume. As a result of water’s polarity, each water molecule attracts. What Absorbs Water Molecules.
From klaefyfol.blob.core.windows.net
How Much Light Does Water Absorb at Taryn McLean blog What Absorbs Water Molecules They grow between soil particles and absorb water and minerals from the soil. Water also attracts or is attracted to other polar molecules and ions. Water is absorbed by root hair cells in plants through osmosis which allows the plant to retain moisture that is needed to survive. As a result of water’s polarity, each water molecule attracts other water. What Absorbs Water Molecules.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID762021 What Absorbs Water Molecules Water's high surface tension is due to the hydrogen bonding in water. When water vapor is absorbed, the water molecules are taken into the molecules of the hygroscopic substance, often resulting in physical changes, such as increased volume. Besides mercury, water has the highest surface tension for all liquids. Water also attracts or is attracted to other polar molecules and. What Absorbs Water Molecules.
From www.youtube.com
Science 4 Q1 Module 1 Lesson 1 Materials that Absorb Water YouTube What Absorbs Water Molecules Water also attracts or is attracted to other polar molecules and ions. As a result of water’s polarity, each water molecule attracts other water molecules because of the opposite charges between water molecules, forming hydrogen bonds. Water enters the root hair cells by osmosis. As a result of water’s polarity, each water molecule attracts other water molecules because of the. What Absorbs Water Molecules.
From cejomfvi.blob.core.windows.net
Importance Of Hydrogen Bonds at Katherine Perez blog What Absorbs Water Molecules Water enters the root hair cells by osmosis. Water is absorbed by root hair cells in plants through osmosis which allows the plant to retain moisture that is needed to survive. Besides mercury, water has the highest surface tension for all liquids. When water vapor is absorbed, the water molecules are taken into the molecules of the hygroscopic substance, often. What Absorbs Water Molecules.
From www.researchgate.net
The mechanism of water absorption on the fibermatrix interface. (a What Absorbs Water Molecules Water enters the root hair cells by osmosis. They grow between soil particles and absorb water and minerals from the soil. Besides mercury, water has the highest surface tension for all liquids. Water's high surface tension is due to the hydrogen bonding in water. Water is absorbed by root hair cells in plants through osmosis which allows the plant to. What Absorbs Water Molecules.
From www.visionlearning.com
Membranes II Biology Visionlearning What Absorbs Water Molecules As a result of water’s polarity, each water molecule attracts other water molecules because of the opposite charges between water molecules, forming hydrogen bonds. Water also attracts or is attracted to other polar molecules and ions. They grow between soil particles and absorb water and minerals from the soil. Besides mercury, water has the highest surface tension for all liquids.. What Absorbs Water Molecules.
From socratic.org
What happens to the energy of its molecules as ice melts into What Absorbs Water Molecules When water vapor is absorbed, the water molecules are taken into the molecules of the hygroscopic substance, often resulting in physical changes, such as increased volume. Water's high surface tension is due to the hydrogen bonding in water. Water is absorbed by root hair cells in plants through osmosis which allows the plant to retain moisture that is needed to. What Absorbs Water Molecules.
From atmos.uw.edu
Latent heat is absorbed or released when water changes phase. What Absorbs Water Molecules Water also attracts or is attracted to other polar molecules and ions. Water's high surface tension is due to the hydrogen bonding in water. They grow between soil particles and absorb water and minerals from the soil. Water enters the root hair cells by osmosis. Besides mercury, water has the highest surface tension for all liquids. As a result of. What Absorbs Water Molecules.
From journals.sagepub.com
Water absorption behavior of cellulosic fibres polymer composites A What Absorbs Water Molecules As a result of water’s polarity, each water molecule attracts other water molecules because of the opposite charges between water molecules, forming hydrogen bonds. As a result of water’s polarity, each water molecule attracts other water molecules because of the opposite charges between water molecules, forming hydrogen bonds. Water also attracts or is attracted to other polar molecules and ions.. What Absorbs Water Molecules.
From www.universallifetools.com
Liquid Crystalline Body Structured Water What Absorbs Water Molecules They grow between soil particles and absorb water and minerals from the soil. Besides mercury, water has the highest surface tension for all liquids. As a result of water’s polarity, each water molecule attracts other water molecules because of the opposite charges between water molecules, forming hydrogen bonds. When water vapor is absorbed, the water molecules are taken into the. What Absorbs Water Molecules.
From klaszcmjb.blob.core.windows.net
How Is Water Bonded Together at Anthony Faison blog What Absorbs Water Molecules Besides mercury, water has the highest surface tension for all liquids. Water enters the root hair cells by osmosis. As a result of water’s polarity, each water molecule attracts other water molecules because of the opposite charges between water molecules, forming hydrogen bonds. As a result of water’s polarity, each water molecule attracts other water molecules because of the opposite. What Absorbs Water Molecules.
From depositphotos.com
Structure Water Molecule Stock Vector by ©AnnSky 574561578 What Absorbs Water Molecules When water vapor is absorbed, the water molecules are taken into the molecules of the hygroscopic substance, often resulting in physical changes, such as increased volume. As a result of water’s polarity, each water molecule attracts other water molecules because of the opposite charges between water molecules, forming hydrogen bonds. Water is absorbed by root hair cells in plants through. What Absorbs Water Molecules.
From www.slideserve.com
PPT Digestion & Absorption PowerPoint Presentation, free download What Absorbs Water Molecules Water also attracts or is attracted to other polar molecules and ions. Water enters the root hair cells by osmosis. Besides mercury, water has the highest surface tension for all liquids. As a result of water’s polarity, each water molecule attracts other water molecules because of the opposite charges between water molecules, forming hydrogen bonds. When water vapor is absorbed,. What Absorbs Water Molecules.
From openoregon.pressbooks.pub
Water Principles of Biology What Absorbs Water Molecules As a result of water’s polarity, each water molecule attracts other water molecules because of the opposite charges between water molecules, forming hydrogen bonds. As a result of water’s polarity, each water molecule attracts other water molecules because of the opposite charges between water molecules, forming hydrogen bonds. Water's high surface tension is due to the hydrogen bonding in water.. What Absorbs Water Molecules.
From www.vedantu.com
In soil, the water available for root absorption is(a) Gravitational What Absorbs Water Molecules Besides mercury, water has the highest surface tension for all liquids. When water vapor is absorbed, the water molecules are taken into the molecules of the hygroscopic substance, often resulting in physical changes, such as increased volume. As a result of water’s polarity, each water molecule attracts other water molecules because of the opposite charges between water molecules, forming hydrogen. What Absorbs Water Molecules.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer What Absorbs Water Molecules When water vapor is absorbed, the water molecules are taken into the molecules of the hygroscopic substance, often resulting in physical changes, such as increased volume. Water is absorbed by root hair cells in plants through osmosis which allows the plant to retain moisture that is needed to survive. Water's high surface tension is due to the hydrogen bonding in. What Absorbs Water Molecules.
From www.udel.edu
Physicists reveal water's secrets in journal 'Science' What Absorbs Water Molecules They grow between soil particles and absorb water and minerals from the soil. As a result of water’s polarity, each water molecule attracts other water molecules because of the opposite charges between water molecules, forming hydrogen bonds. As a result of water’s polarity, each water molecule attracts other water molecules because of the opposite charges between water molecules, forming hydrogen. What Absorbs Water Molecules.
From www.studocu.com
UNIT 10A unit 10 a Water Water is a polar molecule made up of one What Absorbs Water Molecules They grow between soil particles and absorb water and minerals from the soil. As a result of water’s polarity, each water molecule attracts other water molecules because of the opposite charges between water molecules, forming hydrogen bonds. Water's high surface tension is due to the hydrogen bonding in water. When water vapor is absorbed, the water molecules are taken into. What Absorbs Water Molecules.
From www.pinterest.com
// Water Absorption Science Experiment What absorbs water? What does What Absorbs Water Molecules Water is absorbed by root hair cells in plants through osmosis which allows the plant to retain moisture that is needed to survive. Water's high surface tension is due to the hydrogen bonding in water. They grow between soil particles and absorb water and minerals from the soil. When water vapor is absorbed, the water molecules are taken into the. What Absorbs Water Molecules.
From courses.lumenlearning.com
Chemical Digestion and Absorption A Closer Look Anatomy and Physiology What Absorbs Water Molecules Water is absorbed by root hair cells in plants through osmosis which allows the plant to retain moisture that is needed to survive. Water's high surface tension is due to the hydrogen bonding in water. They grow between soil particles and absorb water and minerals from the soil. Water enters the root hair cells by osmosis. As a result of. What Absorbs Water Molecules.
From slidetodoc.com
Superabsorbent Polymer Sodium polyacrylate It is a polymer What Absorbs Water Molecules As a result of water’s polarity, each water molecule attracts other water molecules because of the opposite charges between water molecules, forming hydrogen bonds. Water enters the root hair cells by osmosis. Water's high surface tension is due to the hydrogen bonding in water. Water is absorbed by root hair cells in plants through osmosis which allows the plant to. What Absorbs Water Molecules.
From rwu.pressbooks.pub
Chapter 2. The Chemical Context of Life Introduction to Molecular and What Absorbs Water Molecules As a result of water’s polarity, each water molecule attracts other water molecules because of the opposite charges between water molecules, forming hydrogen bonds. Water is absorbed by root hair cells in plants through osmosis which allows the plant to retain moisture that is needed to survive. As a result of water’s polarity, each water molecule attracts other water molecules. What Absorbs Water Molecules.
From www.dreamstime.com
Water molecule stock illustration. Illustration of letter 24826380 What Absorbs Water Molecules Water also attracts or is attracted to other polar molecules and ions. Water enters the root hair cells by osmosis. Besides mercury, water has the highest surface tension for all liquids. Water's high surface tension is due to the hydrogen bonding in water. When water vapor is absorbed, the water molecules are taken into the molecules of the hygroscopic substance,. What Absorbs Water Molecules.
From punchlistzero.com
Specific Heat of Ice In Various Units, vs. Water, What Absorbs Water Molecules Water is absorbed by root hair cells in plants through osmosis which allows the plant to retain moisture that is needed to survive. Water enters the root hair cells by osmosis. Water's high surface tension is due to the hydrogen bonding in water. Besides mercury, water has the highest surface tension for all liquids. As a result of water’s polarity,. What Absorbs Water Molecules.
From www.worldatlas.com
The Water Cycle WorldAtlas What Absorbs Water Molecules Water is absorbed by root hair cells in plants through osmosis which allows the plant to retain moisture that is needed to survive. They grow between soil particles and absorb water and minerals from the soil. Water also attracts or is attracted to other polar molecules and ions. Water's high surface tension is due to the hydrogen bonding in water.. What Absorbs Water Molecules.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer What Absorbs Water Molecules Water is absorbed by root hair cells in plants through osmosis which allows the plant to retain moisture that is needed to survive. When water vapor is absorbed, the water molecules are taken into the molecules of the hygroscopic substance, often resulting in physical changes, such as increased volume. As a result of water’s polarity, each water molecule attracts other. What Absorbs Water Molecules.
From whatsinsight.org
Does Salt Absorb Water?Simple Explanation What's Insight What Absorbs Water Molecules Water also attracts or is attracted to other polar molecules and ions. Water's high surface tension is due to the hydrogen bonding in water. They grow between soil particles and absorb water and minerals from the soil. Water is absorbed by root hair cells in plants through osmosis which allows the plant to retain moisture that is needed to survive.. What Absorbs Water Molecules.
From www.pinterest.ph
The Fascinating Process of Water Absorption in Plants What Absorbs Water Molecules As a result of water’s polarity, each water molecule attracts other water molecules because of the opposite charges between water molecules, forming hydrogen bonds. Water also attracts or is attracted to other polar molecules and ions. Water's high surface tension is due to the hydrogen bonding in water. As a result of water’s polarity, each water molecule attracts other water. What Absorbs Water Molecules.