Potassium Chloride Lead Nitrate Formula . Example of some compounds that have multiple oxidation states. Note mercury (1) is not a monoatomic cation, but is really a. To start, identify the initial reactants: An aqueous solution containing 8.03g of lead(ii) nitrate is added to an aqueous solution containing 5.26 g of potassium chloride to generate solid. To deduce the formulae of ionic compounds close ionic compound an ionic compound occurs when a negative ion (an atom that has gained an electron). The procedure for naming such compounds is outlined in figure 2.3.1 2.3. We begin with binary ionic compounds, which contain only two elements. The balanced equation will be calculated along with the. 1 and uses the following steps: To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Potassium chloride (kcl) and lead (ii) nitrate (pb(no3)2), and record their states. An alternative way to writing a correct formula for an ionic compound is to use the crisscross method. Enter an equation of an ionic chemical equation and press the balance button. Then, identify the anion and write. In this method, the numerical.
from www.numerade.com
Potassium chloride (kcl) and lead (ii) nitrate (pb(no3)2), and record their states. In this method, the numerical. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. An alternative way to writing a correct formula for an ionic compound is to use the crisscross method. Example of some compounds that have multiple oxidation states. An aqueous solution containing 8.03g of lead(ii) nitrate is added to an aqueous solution containing 5.26 g of potassium chloride to generate solid. Note mercury (1) is not a monoatomic cation, but is really a. To start, identify the initial reactants: To deduce the formulae of ionic compounds close ionic compound an ionic compound occurs when a negative ion (an atom that has gained an electron). Then, identify the anion and write.
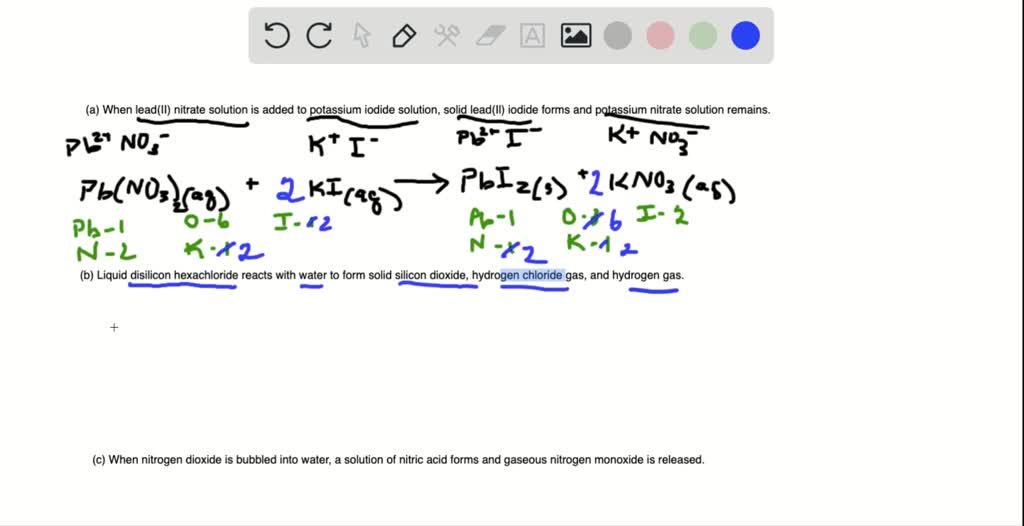
Convert the following into balanced equations (a) When lead(II
Potassium Chloride Lead Nitrate Formula In this method, the numerical. Then, identify the anion and write. Note mercury (1) is not a monoatomic cation, but is really a. In this method, the numerical. We begin with binary ionic compounds, which contain only two elements. An alternative way to writing a correct formula for an ionic compound is to use the crisscross method. 1 and uses the following steps: Potassium chloride (kcl) and lead (ii) nitrate (pb(no3)2), and record their states. Example of some compounds that have multiple oxidation states. To start, identify the initial reactants: The procedure for naming such compounds is outlined in figure 2.3.1 2.3. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. To deduce the formulae of ionic compounds close ionic compound an ionic compound occurs when a negative ion (an atom that has gained an electron). An aqueous solution containing 8.03g of lead(ii) nitrate is added to an aqueous solution containing 5.26 g of potassium chloride to generate solid. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge.
From brainly.in
Sodium chloride solution is added to lead nitrate solution. Also write Potassium Chloride Lead Nitrate Formula The procedure for naming such compounds is outlined in figure 2.3.1 2.3. Example of some compounds that have multiple oxidation states. Potassium chloride (kcl) and lead (ii) nitrate (pb(no3)2), and record their states. An alternative way to writing a correct formula for an ionic compound is to use the crisscross method. Enter an equation of an ionic chemical equation and. Potassium Chloride Lead Nitrate Formula.
From www.chegg.com
Solved An aqueous solution containing 5.01 g of lead(II) Potassium Chloride Lead Nitrate Formula To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Potassium chloride (kcl) and lead (ii) nitrate (pb(no3)2), and record their states. An aqueous solution containing 8.03g of lead(ii) nitrate is added to an aqueous solution containing 5.26 g of potassium chloride to generate solid. Then, identify the anion and write.. Potassium Chloride Lead Nitrate Formula.
From www.teachoo.com
Case Based Class 10 Science The physical states of the reactants Potassium Chloride Lead Nitrate Formula In this method, the numerical. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. To deduce the formulae of ionic compounds close ionic compound an ionic compound occurs when a negative ion (an atom that has gained an electron). 1 and uses the following steps: Potassium chloride (kcl) and lead. Potassium Chloride Lead Nitrate Formula.
From chemistry.stackexchange.com
chemistry Solubility of potassium nitrate and potassium Potassium Chloride Lead Nitrate Formula To start, identify the initial reactants: In this method, the numerical. Example of some compounds that have multiple oxidation states. Enter an equation of an ionic chemical equation and press the balance button. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. The balanced equation will be calculated along with. Potassium Chloride Lead Nitrate Formula.
From lauriekyrran.blogspot.com
Potassium Hydroxide And Iron Ii Nitrate Precipitate LaurieKyrran Potassium Chloride Lead Nitrate Formula An alternative way to writing a correct formula for an ionic compound is to use the crisscross method. We begin with binary ionic compounds, which contain only two elements. To start, identify the initial reactants: To deduce the formulae of ionic compounds close ionic compound an ionic compound occurs when a negative ion (an atom that has gained an electron).. Potassium Chloride Lead Nitrate Formula.
From yazmingokefoster.blogspot.com
Particle Diagram of Lead Nitrate and Potassium Iodide Potassium Chloride Lead Nitrate Formula Note mercury (1) is not a monoatomic cation, but is really a. The procedure for naming such compounds is outlined in figure 2.3.1 2.3. An aqueous solution containing 8.03g of lead(ii) nitrate is added to an aqueous solution containing 5.26 g of potassium chloride to generate solid. Example of some compounds that have multiple oxidation states. In this method, the. Potassium Chloride Lead Nitrate Formula.
From www.slideserve.com
PPT Solubility Rules PowerPoint Presentation, free download ID4934641 Potassium Chloride Lead Nitrate Formula Potassium chloride (kcl) and lead (ii) nitrate (pb(no3)2), and record their states. The procedure for naming such compounds is outlined in figure 2.3.1 2.3. To deduce the formulae of ionic compounds close ionic compound an ionic compound occurs when a negative ion (an atom that has gained an electron). Example of some compounds that have multiple oxidation states. Enter an. Potassium Chloride Lead Nitrate Formula.
From www.youtube.com
007 Potassium Iodide + Lead (II) Nitrate = Lead (II) Iodide Potassium Chloride Lead Nitrate Formula An aqueous solution containing 8.03g of lead(ii) nitrate is added to an aqueous solution containing 5.26 g of potassium chloride to generate solid. To start, identify the initial reactants: To deduce the formulae of ionic compounds close ionic compound an ionic compound occurs when a negative ion (an atom that has gained an electron). An alternative way to writing a. Potassium Chloride Lead Nitrate Formula.
From www.numerade.com
SOLVED Write balanced complete chemical equation the complete ionic Potassium Chloride Lead Nitrate Formula Potassium chloride (kcl) and lead (ii) nitrate (pb(no3)2), and record their states. An aqueous solution containing 8.03g of lead(ii) nitrate is added to an aqueous solution containing 5.26 g of potassium chloride to generate solid. To start, identify the initial reactants: To find the formula of an ionic compound, first identify the cation and write down its symbol and charge.. Potassium Chloride Lead Nitrate Formula.
From protonstalk.com
Lead Nitrate Formula, Structure, Preparation & Properties ProtonsTalk Potassium Chloride Lead Nitrate Formula We begin with binary ionic compounds, which contain only two elements. In this method, the numerical. Then, identify the anion and write. An aqueous solution containing 8.03g of lead(ii) nitrate is added to an aqueous solution containing 5.26 g of potassium chloride to generate solid. Note mercury (1) is not a monoatomic cation, but is really a. Potassium chloride (kcl). Potassium Chloride Lead Nitrate Formula.
From toppr.com
\"Reaction of potassium iodide solution with lead nitrate solution Potassium Chloride Lead Nitrate Formula Then, identify the anion and write. The procedure for naming such compounds is outlined in figure 2.3.1 2.3. An aqueous solution containing 8.03g of lead(ii) nitrate is added to an aqueous solution containing 5.26 g of potassium chloride to generate solid. To deduce the formulae of ionic compounds close ionic compound an ionic compound occurs when a negative ion (an. Potassium Chloride Lead Nitrate Formula.
From www.youtube.com
Predict the Products Pb(NO3)2 + KI Lead (II) Nitrate + Potassium Potassium Chloride Lead Nitrate Formula An aqueous solution containing 8.03g of lead(ii) nitrate is added to an aqueous solution containing 5.26 g of potassium chloride to generate solid. The procedure for naming such compounds is outlined in figure 2.3.1 2.3. Example of some compounds that have multiple oxidation states. To deduce the formulae of ionic compounds close ionic compound an ionic compound occurs when a. Potassium Chloride Lead Nitrate Formula.
From www.youtube.com
IGCSE Chemistry lesson 34 part c Thermal of nitrates Potassium Chloride Lead Nitrate Formula We begin with binary ionic compounds, which contain only two elements. An aqueous solution containing 8.03g of lead(ii) nitrate is added to an aqueous solution containing 5.26 g of potassium chloride to generate solid. To deduce the formulae of ionic compounds close ionic compound an ionic compound occurs when a negative ion (an atom that has gained an electron). 1. Potassium Chloride Lead Nitrate Formula.
From www.numerade.com
SOLVED Write a balanced equation for the reaction between aqueous lead Potassium Chloride Lead Nitrate Formula The balanced equation will be calculated along with the. Note mercury (1) is not a monoatomic cation, but is really a. Potassium chloride (kcl) and lead (ii) nitrate (pb(no3)2), and record their states. Enter an equation of an ionic chemical equation and press the balance button. In this method, the numerical. An aqueous solution containing 8.03g of lead(ii) nitrate is. Potassium Chloride Lead Nitrate Formula.
From www.numerade.com
Convert the following into balanced equations (a) When lead(II Potassium Chloride Lead Nitrate Formula The procedure for naming such compounds is outlined in figure 2.3.1 2.3. Example of some compounds that have multiple oxidation states. In this method, the numerical. To start, identify the initial reactants: An alternative way to writing a correct formula for an ionic compound is to use the crisscross method. The balanced equation will be calculated along with the. Enter. Potassium Chloride Lead Nitrate Formula.
From joicffnpl.blob.core.windows.net
Lead Ii Nitrate Reaction With Potassium Iodide at Matilda Lee blog Potassium Chloride Lead Nitrate Formula Enter an equation of an ionic chemical equation and press the balance button. An alternative way to writing a correct formula for an ionic compound is to use the crisscross method. Potassium chloride (kcl) and lead (ii) nitrate (pb(no3)2), and record their states. Then, identify the anion and write. To deduce the formulae of ionic compounds close ionic compound an. Potassium Chloride Lead Nitrate Formula.
From www.teachoo.com
MCQ Reema took 5ml of Lead Nitrate solution in a beaker and added ap Potassium Chloride Lead Nitrate Formula The procedure for naming such compounds is outlined in figure 2.3.1 2.3. Example of some compounds that have multiple oxidation states. The balanced equation will be calculated along with the. Potassium chloride (kcl) and lead (ii) nitrate (pb(no3)2), and record their states. Note mercury (1) is not a monoatomic cation, but is really a. In this method, the numerical. Then,. Potassium Chloride Lead Nitrate Formula.
From slideplayer.com
Precipitation Reactions ppt download Potassium Chloride Lead Nitrate Formula Then, identify the anion and write. Note mercury (1) is not a monoatomic cation, but is really a. Potassium chloride (kcl) and lead (ii) nitrate (pb(no3)2), and record their states. In this method, the numerical. An alternative way to writing a correct formula for an ionic compound is to use the crisscross method. To deduce the formulae of ionic compounds. Potassium Chloride Lead Nitrate Formula.
From www.nagwa.com
Question Video Determining the Mass of the Potassium Chloride Analyte Potassium Chloride Lead Nitrate Formula Enter an equation of an ionic chemical equation and press the balance button. To deduce the formulae of ionic compounds close ionic compound an ionic compound occurs when a negative ion (an atom that has gained an electron). Potassium chloride (kcl) and lead (ii) nitrate (pb(no3)2), and record their states. 1 and uses the following steps: To find the formula. Potassium Chloride Lead Nitrate Formula.
From brainly.in
Show me the formula of following salt by criss cross method 1 Potassium Chloride Lead Nitrate Formula To start, identify the initial reactants: In this method, the numerical. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. Note mercury (1) is not a monoatomic cation,. Potassium Chloride Lead Nitrate Formula.
From www.youtube.com
How to Balance Pb(NO3)2 + NaCl = PbCl2 + NaNO3 YouTube Potassium Chloride Lead Nitrate Formula The balanced equation will be calculated along with the. In this method, the numerical. To start, identify the initial reactants: 1 and uses the following steps: The procedure for naming such compounds is outlined in figure 2.3.1 2.3. Enter an equation of an ionic chemical equation and press the balance button. Potassium chloride (kcl) and lead (ii) nitrate (pb(no3)2), and. Potassium Chloride Lead Nitrate Formula.
From www.bartleby.com
Answered When aqueous solutions of iron(II)… bartleby Potassium Chloride Lead Nitrate Formula An alternative way to writing a correct formula for an ionic compound is to use the crisscross method. Then, identify the anion and write. Potassium chloride (kcl) and lead (ii) nitrate (pb(no3)2), and record their states. In this method, the numerical. The balanced equation will be calculated along with the. To start, identify the initial reactants: Example of some compounds. Potassium Chloride Lead Nitrate Formula.
From brainly.in
With the help of criss cross method write the chemical formula of Potassium Chloride Lead Nitrate Formula The procedure for naming such compounds is outlined in figure 2.3.1 2.3. Note mercury (1) is not a monoatomic cation, but is really a. Then, identify the anion and write. Enter an equation of an ionic chemical equation and press the balance button. An alternative way to writing a correct formula for an ionic compound is to use the crisscross. Potassium Chloride Lead Nitrate Formula.
From www.dreamstime.com
Potassium Chloride Chemical Formula on Waterdrop Background Stock Potassium Chloride Lead Nitrate Formula The balanced equation will be calculated along with the. In this method, the numerical. 1 and uses the following steps: Then, identify the anion and write. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Potassium chloride (kcl) and lead (ii) nitrate (pb(no3)2), and record their states. An alternative way. Potassium Chloride Lead Nitrate Formula.
From loepbliqd.blob.core.windows.net
Lead Ii Nitrate Sodium Carbonate Ionic Equation at Mitchell Laxton blog Potassium Chloride Lead Nitrate Formula The procedure for naming such compounds is outlined in figure 2.3.1 2.3. The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. Note mercury (1) is not a monoatomic cation, but is really a. To start, identify the initial reactants: An aqueous solution containing 8.03g of lead(ii) nitrate. Potassium Chloride Lead Nitrate Formula.
From www.youtube.com
Experiment on Lead nitrate and Potassium iodide I Class 10 Chemistry Potassium Chloride Lead Nitrate Formula Potassium chloride (kcl) and lead (ii) nitrate (pb(no3)2), and record their states. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Then, identify the anion and write. Enter an equation of an ionic chemical equation and press the balance button. Note mercury (1) is not a monoatomic cation, but is. Potassium Chloride Lead Nitrate Formula.
From loepbliqd.blob.core.windows.net
Lead Ii Nitrate Sodium Carbonate Ionic Equation at Mitchell Laxton blog Potassium Chloride Lead Nitrate Formula Example of some compounds that have multiple oxidation states. Potassium chloride (kcl) and lead (ii) nitrate (pb(no3)2), and record their states. Enter an equation of an ionic chemical equation and press the balance button. The procedure for naming such compounds is outlined in figure 2.3.1 2.3. To deduce the formulae of ionic compounds close ionic compound an ionic compound occurs. Potassium Chloride Lead Nitrate Formula.
From www.numerade.com
SOLVED Aqueous lead (II) nitrate, Pb(NO3)2 undergoes a double Potassium Chloride Lead Nitrate Formula The procedure for naming such compounds is outlined in figure 2.3.1 2.3. To start, identify the initial reactants: To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. An alternative way to writing a correct formula for an ionic compound is to use the crisscross method. Then, identify the anion and. Potassium Chloride Lead Nitrate Formula.
From www.chegg.com
Solved 6. Potassium Chloride & Silver (1) Nitrate Equation Potassium Chloride Lead Nitrate Formula To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. An aqueous solution containing 8.03g of lead(ii) nitrate is added to an aqueous solution containing 5.26 g of potassium chloride to generate solid. In this method, the numerical. Example of some compounds that have multiple oxidation states. To start, identify the. Potassium Chloride Lead Nitrate Formula.
From www.numerade.com
SOLVED Aqueous ammonium chloride and aqueous lead (II) nitrate react Potassium Chloride Lead Nitrate Formula Potassium chloride (kcl) and lead (ii) nitrate (pb(no3)2), and record their states. To start, identify the initial reactants: To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Example of some compounds that have multiple oxidation states. We begin with binary ionic compounds, which contain only two elements. The procedure for. Potassium Chloride Lead Nitrate Formula.
From www.chegg.com
Solved Complete the table below by deciding whether a Potassium Chloride Lead Nitrate Formula Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. The procedure for naming such compounds is outlined in figure 2.3.1 2.3. Potassium chloride (kcl) and lead (ii) nitrate (pb(no3)2), and record their states. 1 and uses the following steps: An alternative way to writing a correct formula. Potassium Chloride Lead Nitrate Formula.
From askfilo.com
What is the Chemical formula of? lead Nitrate ii) Potassium lodide ii) Le.. Potassium Chloride Lead Nitrate Formula Enter an equation of an ionic chemical equation and press the balance button. To start, identify the initial reactants: The balanced equation will be calculated along with the. Potassium chloride (kcl) and lead (ii) nitrate (pb(no3)2), and record their states. In this method, the numerical. 1 and uses the following steps: An alternative way to writing a correct formula for. Potassium Chloride Lead Nitrate Formula.
From www.numerade.com
SOLVED 'Identify the ionic and covalent bond in the following Potassium Chloride Lead Nitrate Formula The procedure for naming such compounds is outlined in figure 2.3.1 2.3. An aqueous solution containing 8.03g of lead(ii) nitrate is added to an aqueous solution containing 5.26 g of potassium chloride to generate solid. An alternative way to writing a correct formula for an ionic compound is to use the crisscross method. We begin with binary ionic compounds, which. Potassium Chloride Lead Nitrate Formula.
From www.slideserve.com
PPT Chapter 4 Chemical Reactions An Introduction PowerPoint Potassium Chloride Lead Nitrate Formula In this method, the numerical. Note mercury (1) is not a monoatomic cation, but is really a. Then, identify the anion and write. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Example of some compounds that have multiple oxidation states. We begin with binary ionic compounds, which contain only. Potassium Chloride Lead Nitrate Formula.
From wisc.pb.unizin.org
Solutions and Solubility (part 2) (M3Q2) UWMadison Chemistry 103/104 Potassium Chloride Lead Nitrate Formula To start, identify the initial reactants: Enter an equation of an ionic chemical equation and press the balance button. In this method, the numerical. Potassium chloride (kcl) and lead (ii) nitrate (pb(no3)2), and record their states. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. We begin with binary ionic. Potassium Chloride Lead Nitrate Formula.