When Heat Is Absorbed . Q means the heat absorbed, m is the mass of the substance absorbing heat, c is the specific heat capacity and. In both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite in sign to the amount of heat. Heat capacity is the amount of heat necessary to change the temperature of a. Heat absorbed by a system is positive (q > 0), indicating that the. Heat waves radiate out from hot objects in all directions, travelling at the speed of light, until they hit another object. When heat is transferred to an object by its surroundings, then the object can warm up and the surroundings can cool down. When this happens, the heat energy carried by the waves can be either. Specific heat is the amount of. Calculate heat absorption using the formula: Heat, once absorbed as energy, contributes to the overall. What are good and bad absorbers of heat? Specific heat is closely related to the concept of heat capacity. This specific heat calculator is a tool that determines the heat capacity of a heated or a cooled sample. How does the heat travel between objects? In this physics tutorial, you will learn:
from www.doubtnut.com
Specific heat is closely related to the concept of heat capacity. Q means the heat absorbed, m is the mass of the substance absorbing heat, c is the specific heat capacity and. Calculate heat absorption using the formula: Q = mc ∆ t. Heat capacity is the amount of heat necessary to change the temperature of a. In physics equations, the sign convention for heat transfer indicates the direction of energy flow. This specific heat calculator is a tool that determines the heat capacity of a heated or a cooled sample. Heat waves radiate out from hot objects in all directions, travelling at the speed of light, until they hit another object. What are good and bad absorbers of heat? Heat, once absorbed as energy, contributes to the overall.
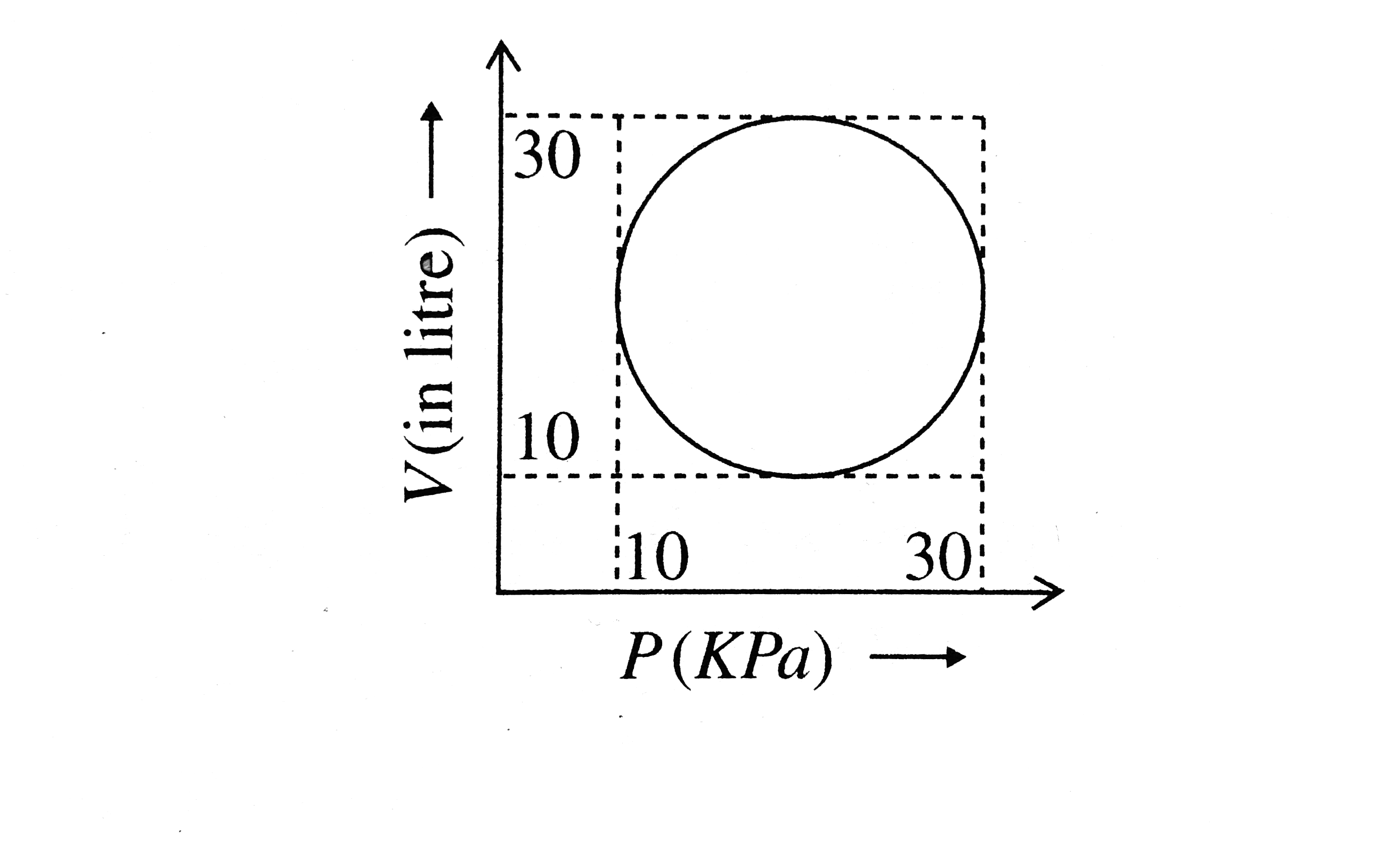
Heat energy absorbed by a system in going through a cyclic process sho
When Heat Is Absorbed When heat is transferred to an object by its surroundings, then the object can warm up and the surroundings can cool down. Specific heat is closely related to the concept of heat capacity. Heat, once absorbed as energy, contributes to the overall. In both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite in sign to the amount of heat. This specific heat calculator is a tool that determines the heat capacity of a heated or a cooled sample. Q means the heat absorbed, m is the mass of the substance absorbing heat, c is the specific heat capacity and. What are good and bad absorbers of heat? Specific heat is the amount of. Heat absorbed by a system is positive (q > 0), indicating that the. In physics equations, the sign convention for heat transfer indicates the direction of energy flow. When heat is transferred to an object by its surroundings, then the object can warm up and the surroundings can cool down. Q = mc ∆ t. Calculate heat absorption using the formula: In this physics tutorial, you will learn: Heat capacity is the amount of heat necessary to change the temperature of a. When this happens, the heat energy carried by the waves can be either.
From www.youtube.com
Phase Changes Energy changes and Latent Heat YouTube When Heat Is Absorbed Heat, once absorbed as energy, contributes to the overall. Heat capacity is the amount of heat necessary to change the temperature of a. Specific heat is closely related to the concept of heat capacity. What are good and bad absorbers of heat? In physics equations, the sign convention for heat transfer indicates the direction of energy flow. In both cases,. When Heat Is Absorbed.
From www.slideserve.com
PPT Chapter 6 Chemical Reactions and Quantities PowerPoint When Heat Is Absorbed Heat capacity is the amount of heat necessary to change the temperature of a. What are good and bad absorbers of heat? Calculate heat absorption using the formula: In this physics tutorial, you will learn: Heat, once absorbed as energy, contributes to the overall. Q = mc ∆ t. Specific heat is the amount of. When heat is transferred to. When Heat Is Absorbed.
From www.pinterest.com
Latent Heat the heat energy that must be absorbed when a substance When Heat Is Absorbed Specific heat is the amount of. When this happens, the heat energy carried by the waves can be either. Heat absorbed by a system is positive (q > 0), indicating that the. Q = mc ∆ t. Heat, once absorbed as energy, contributes to the overall. In both cases, the amount of heat absorbed or released by the calorimeter is. When Heat Is Absorbed.
From slideplayer.com
Unit 3 Topic ppt download When Heat Is Absorbed Calculate heat absorption using the formula: Heat capacity is the amount of heat necessary to change the temperature of a. Specific heat is the amount of. In both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite in sign to the amount of heat. Heat, once absorbed as energy, contributes to the. When Heat Is Absorbed.
From www.vrogue.co
Heat Absorbed Formulas How To Calculate It And Solved vrogue.co When Heat Is Absorbed Q = mc ∆ t. This specific heat calculator is a tool that determines the heat capacity of a heated or a cooled sample. How does the heat travel between objects? When heat is transferred to an object by its surroundings, then the object can warm up and the surroundings can cool down. Specific heat is the amount of. Heat,. When Heat Is Absorbed.
From www.youtube.com
Heat Absorbed During a Reaction (Example) YouTube When Heat Is Absorbed This specific heat calculator is a tool that determines the heat capacity of a heated or a cooled sample. In both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite in sign to the amount of heat. How does the heat travel between objects? Specific heat is closely related to the concept. When Heat Is Absorbed.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation When Heat Is Absorbed In physics equations, the sign convention for heat transfer indicates the direction of energy flow. Specific heat is closely related to the concept of heat capacity. In this physics tutorial, you will learn: In both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite in sign to the amount of heat. Q. When Heat Is Absorbed.
From brainly.in
In the diagram below, label all of the arrows as either Heat Released When Heat Is Absorbed How does the heat travel between objects? Specific heat is the amount of. In this physics tutorial, you will learn: When this happens, the heat energy carried by the waves can be either. Heat, once absorbed as energy, contributes to the overall. Specific heat is closely related to the concept of heat capacity. Heat waves radiate out from hot objects. When Heat Is Absorbed.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science When Heat Is Absorbed Specific heat is closely related to the concept of heat capacity. Q means the heat absorbed, m is the mass of the substance absorbing heat, c is the specific heat capacity and. In physics equations, the sign convention for heat transfer indicates the direction of energy flow. In both cases, the amount of heat absorbed or released by the calorimeter. When Heat Is Absorbed.
From www.doubtnut.com
Heat energy absorbed by a system in going through a cyclic process sho When Heat Is Absorbed Specific heat is the amount of. Calculate heat absorption using the formula: In both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite in sign to the amount of heat. Q means the heat absorbed, m is the mass of the substance absorbing heat, c is the specific heat capacity and. Specific. When Heat Is Absorbed.
From www.slideserve.com
PPT Heat (q) PowerPoint Presentation, free download ID1551407 When Heat Is Absorbed When this happens, the heat energy carried by the waves can be either. This specific heat calculator is a tool that determines the heat capacity of a heated or a cooled sample. Specific heat is closely related to the concept of heat capacity. Q means the heat absorbed, m is the mass of the substance absorbing heat, c is the. When Heat Is Absorbed.
From www.youtube.com
3.2a Heating the Atmosphere YouTube When Heat Is Absorbed Heat absorbed by a system is positive (q > 0), indicating that the. In both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite in sign to the amount of heat. Heat waves radiate out from hot objects in all directions, travelling at the speed of light, until they hit another object.. When Heat Is Absorbed.
From www.slideserve.com
PPT Ch. 2 Matter and Energy PowerPoint Presentation, free download When Heat Is Absorbed Q = mc ∆ t. In both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite in sign to the amount of heat. How does the heat travel between objects? Calculate heat absorption using the formula: When this happens, the heat energy carried by the waves can be either. Heat, once absorbed. When Heat Is Absorbed.
From www.ces.fau.edu
Climate Science Investigations South Florida Energy The Driver of When Heat Is Absorbed Heat capacity is the amount of heat necessary to change the temperature of a. Q means the heat absorbed, m is the mass of the substance absorbing heat, c is the specific heat capacity and. Specific heat is the amount of. Heat absorbed by a system is positive (q > 0), indicating that the. In physics equations, the sign convention. When Heat Is Absorbed.
From www.processtechacademy.com
Proc Tech & Oper Acad Sensible & Latent Heat When Heat Is Absorbed Heat waves radiate out from hot objects in all directions, travelling at the speed of light, until they hit another object. Q means the heat absorbed, m is the mass of the substance absorbing heat, c is the specific heat capacity and. In both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and. When Heat Is Absorbed.
From www.slideserve.com
PPT The Earth’s Global Energy Balance PowerPoint Presentation, free When Heat Is Absorbed How does the heat travel between objects? When heat is transferred to an object by its surroundings, then the object can warm up and the surroundings can cool down. Q means the heat absorbed, m is the mass of the substance absorbing heat, c is the specific heat capacity and. When this happens, the heat energy carried by the waves. When Heat Is Absorbed.
From www.slideshare.net
CHAPTER FIFTEEN Transmission of Heat Energy When Heat Is Absorbed What are good and bad absorbers of heat? In both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite in sign to the amount of heat. When this happens, the heat energy carried by the waves can be either. This specific heat calculator is a tool that determines the heat capacity of. When Heat Is Absorbed.
From www.bigstockphoto.com
Light Absorption Image & Photo (Free Trial) Bigstock When Heat Is Absorbed Q = mc ∆ t. This specific heat calculator is a tool that determines the heat capacity of a heated or a cooled sample. How does the heat travel between objects? Calculate heat absorption using the formula: Heat, once absorbed as energy, contributes to the overall. When this happens, the heat energy carried by the waves can be either. In. When Heat Is Absorbed.
From earthscience.xyz
Heat Transfer Earth Science When Heat Is Absorbed How does the heat travel between objects? When this happens, the heat energy carried by the waves can be either. In physics equations, the sign convention for heat transfer indicates the direction of energy flow. In this physics tutorial, you will learn: Heat absorbed by a system is positive (q > 0), indicating that the. This specific heat calculator is. When Heat Is Absorbed.
From spmchemistry.blog.onlinetuition.com.my
Heat of Combustion SPM Chemistry When Heat Is Absorbed When this happens, the heat energy carried by the waves can be either. Specific heat is closely related to the concept of heat capacity. Calculate heat absorption using the formula: Heat absorbed by a system is positive (q > 0), indicating that the. In this physics tutorial, you will learn: What are good and bad absorbers of heat? Q =. When Heat Is Absorbed.
From conceptgroupllc.com
What is phase change? Explained by Thermal Engineers When Heat Is Absorbed Q = mc ∆ t. Q means the heat absorbed, m is the mass of the substance absorbing heat, c is the specific heat capacity and. Calculate heat absorption using the formula: Specific heat is the amount of. What are good and bad absorbers of heat? Heat, once absorbed as energy, contributes to the overall. Specific heat is closely related. When Heat Is Absorbed.
From www.researchgate.net
3 Heat energy absorbed and released Download Scientific Diagram When Heat Is Absorbed When this happens, the heat energy carried by the waves can be either. When heat is transferred to an object by its surroundings, then the object can warm up and the surroundings can cool down. This specific heat calculator is a tool that determines the heat capacity of a heated or a cooled sample. Heat absorbed by a system is. When Heat Is Absorbed.
From www.slideshare.net
Heat When Heat Is Absorbed Heat capacity is the amount of heat necessary to change the temperature of a. What are good and bad absorbers of heat? In this physics tutorial, you will learn: Specific heat is closely related to the concept of heat capacity. In physics equations, the sign convention for heat transfer indicates the direction of energy flow. This specific heat calculator is. When Heat Is Absorbed.
From www.flinnsci.com
360 Science Thermal Energy and Heat Transfer When Heat Is Absorbed In both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite in sign to the amount of heat. What are good and bad absorbers of heat? Calculate heat absorption using the formula: Q = mc ∆ t. Specific heat is closely related to the concept of heat capacity. Heat capacity is the. When Heat Is Absorbed.
From www.chemistrylearner.com
Heat (Enthalpy) of Solution Definition, Formula, & Problems When Heat Is Absorbed Heat absorbed by a system is positive (q > 0), indicating that the. Heat, once absorbed as energy, contributes to the overall. When heat is transferred to an object by its surroundings, then the object can warm up and the surroundings can cool down. This specific heat calculator is a tool that determines the heat capacity of a heated or. When Heat Is Absorbed.
From www.carbonbrief.org
Heat absorbed by oceans has doubled since 1997 Carbon Brief When Heat Is Absorbed This specific heat calculator is a tool that determines the heat capacity of a heated or a cooled sample. When this happens, the heat energy carried by the waves can be either. When heat is transferred to an object by its surroundings, then the object can warm up and the surroundings can cool down. Q means the heat absorbed, m. When Heat Is Absorbed.
From sciencenotes.org
Endothermic Reactions Definition and Examples When Heat Is Absorbed Calculate heat absorption using the formula: Heat, once absorbed as energy, contributes to the overall. Specific heat is the amount of. In physics equations, the sign convention for heat transfer indicates the direction of energy flow. What are good and bad absorbers of heat? Q = mc ∆ t. Specific heat is closely related to the concept of heat capacity.. When Heat Is Absorbed.
From opentextbc.ca
Phase Changes Basic HVAC When Heat Is Absorbed In both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite in sign to the amount of heat. When heat is transferred to an object by its surroundings, then the object can warm up and the surroundings can cool down. Q = mc ∆ t. This specific heat calculator is a tool. When Heat Is Absorbed.
From general.chemistrysteps.com
What is Enthalpy Chemistry Steps When Heat Is Absorbed Specific heat is the amount of. Q = mc ∆ t. What are good and bad absorbers of heat? Heat absorbed by a system is positive (q > 0), indicating that the. In this physics tutorial, you will learn: When heat is transferred to an object by its surroundings, then the object can warm up and the surroundings can cool. When Heat Is Absorbed.
From www.slideserve.com
PPT Enthalpy is the heat absorbed or released by a system at When Heat Is Absorbed Heat, once absorbed as energy, contributes to the overall. Heat waves radiate out from hot objects in all directions, travelling at the speed of light, until they hit another object. This specific heat calculator is a tool that determines the heat capacity of a heated or a cooled sample. Calculate heat absorption using the formula: How does the heat travel. When Heat Is Absorbed.
From studylib.net
Heat Absorbed by Ice Heat Transferred from Water When Heat Is Absorbed How does the heat travel between objects? Q = mc ∆ t. In both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite in sign to the amount of heat. Heat absorbed by a system is positive (q > 0), indicating that the. Calculate heat absorption using the formula: What are good. When Heat Is Absorbed.
From www.youtube.com
Week 1 16. How to calculate the heat absorbed when a substance When Heat Is Absorbed Specific heat is the amount of. Heat absorbed by a system is positive (q > 0), indicating that the. Heat, once absorbed as energy, contributes to the overall. In both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite in sign to the amount of heat. This specific heat calculator is a. When Heat Is Absorbed.
From worksheetfullfunniest.z21.web.core.windows.net
Heat Energy During Phase Change When Heat Is Absorbed When this happens, the heat energy carried by the waves can be either. This specific heat calculator is a tool that determines the heat capacity of a heated or a cooled sample. What are good and bad absorbers of heat? Specific heat is closely related to the concept of heat capacity. When heat is transferred to an object by its. When Heat Is Absorbed.
From sites.google.com
Heat Transfers Solar Cooker Challenge When Heat Is Absorbed In physics equations, the sign convention for heat transfer indicates the direction of energy flow. In this physics tutorial, you will learn: In both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite in sign to the amount of heat. Calculate heat absorption using the formula: What are good and bad absorbers. When Heat Is Absorbed.
From spectacularsci.com
What Are The 3 Types of Heat Transfer? Spectacular Science When Heat Is Absorbed Heat, once absorbed as energy, contributes to the overall. Heat absorbed by a system is positive (q > 0), indicating that the. When this happens, the heat energy carried by the waves can be either. Heat waves radiate out from hot objects in all directions, travelling at the speed of light, until they hit another object. Q means the heat. When Heat Is Absorbed.