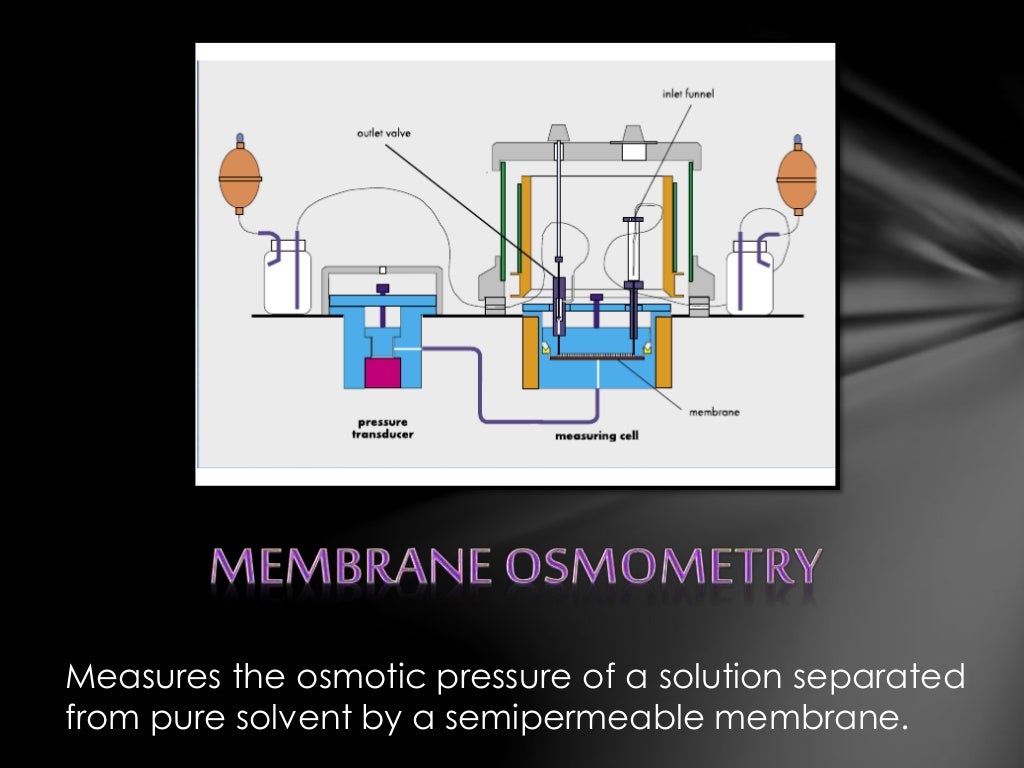
Osmometry Test . An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar. Freezing point depression osmometers are utilized to determine a solution's osmotic strength. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: Osmolality testing uses state of the art pieces of laboratory instruments known as osmometers. Osmometry can be expressed in two ways:. It is the approach that is most. Osmolarity and osmolality are ways to measure and report the osmometry of a solvent. Osmometry plays a key role in determining molecular weights of solutes based on colligative properties like vapor pressure lowering and. Clinical labs use osmometers to test osmolality of body fluids including but not limited to plasma, serum and urine for diagnostic purposes and for. There are two main types, vapor pressure.
from www.slideshare.net
It is the approach that is most. Osmometry can be expressed in two ways:. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: Osmolality testing uses state of the art pieces of laboratory instruments known as osmometers. Osmometry plays a key role in determining molecular weights of solutes based on colligative properties like vapor pressure lowering and. Osmolarity and osmolality are ways to measure and report the osmometry of a solvent. Clinical labs use osmometers to test osmolality of body fluids including but not limited to plasma, serum and urine for diagnostic purposes and for. There are two main types, vapor pressure. Freezing point depression osmometers are utilized to determine a solution's osmotic strength. An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar.
Osmometry report.pdf
Osmometry Test There are two main types, vapor pressure. Osmolality testing uses state of the art pieces of laboratory instruments known as osmometers. Osmolarity and osmolality are ways to measure and report the osmometry of a solvent. It is the approach that is most. Osmometry plays a key role in determining molecular weights of solutes based on colligative properties like vapor pressure lowering and. Clinical labs use osmometers to test osmolality of body fluids including but not limited to plasma, serum and urine for diagnostic purposes and for. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: Osmometry can be expressed in two ways:. Freezing point depression osmometers are utilized to determine a solution's osmotic strength. There are two main types, vapor pressure. An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar.
From www.tbs.ge
HPLC UHPLC Osmometry & etc. Osmometry Test Osmometry can be expressed in two ways:. Osmolality testing uses state of the art pieces of laboratory instruments known as osmometers. An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar. Osmolarity and osmolality are ways to measure and report the osmometry of a solvent. Osmometry plays a. Osmometry Test.
From www.youtube.com
Osmometry YouTube Osmometry Test Freezing point depression osmometers are utilized to determine a solution's osmotic strength. Osmometry can be expressed in two ways:. Osmolarity and osmolality are ways to measure and report the osmometry of a solvent. It is the approach that is most. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: Clinical labs use. Osmometry Test.
From www.geminibv.fr
Advanced instruments inc. 3300 Micro Osmomètre Gemini BV Osmometry Test There are two main types, vapor pressure. Clinical labs use osmometers to test osmolality of body fluids including but not limited to plasma, serum and urine for diagnostic purposes and for. Osmolality testing uses state of the art pieces of laboratory instruments known as osmometers. Osmolarity and osmolality are ways to measure and report the osmometry of a solvent. An. Osmometry Test.
From www.biophlox.com
A Complete Lab Guide to Osmometer Osmometry Test Osmometry can be expressed in two ways:. Clinical labs use osmometers to test osmolality of body fluids including but not limited to plasma, serum and urine for diagnostic purposes and for. Osmometry plays a key role in determining molecular weights of solutes based on colligative properties like vapor pressure lowering and. Osmolarity and osmolality are ways to measure and report. Osmometry Test.
From www.mrclab.com
OSMOMETER single sample Osmometry Test There are two main types, vapor pressure. Osmolality testing uses state of the art pieces of laboratory instruments known as osmometers. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar.. Osmometry Test.
From www.scribd.com
Vapor Pressure Osmometry PDF Osmometry Test Osmometry plays a key role in determining molecular weights of solutes based on colligative properties like vapor pressure lowering and. Clinical labs use osmometers to test osmolality of body fluids including but not limited to plasma, serum and urine for diagnostic purposes and for. There are two main types, vapor pressure. There are two principal methods of osmometry that are. Osmometry Test.
From www.flickriver.com
Membrane Osmometry a photo on Flickriver Osmometry Test There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: Osmometry can be expressed in two ways:. There are two main types, vapor pressure. Clinical labs use osmometers to test osmolality of body fluids including but not limited to plasma, serum and urine for diagnostic purposes and for. An osmometer is a device. Osmometry Test.
From www.il-biosystems.com
Advanced Instruments OsmoPRO MAX Osmometer Osmometry Test Clinical labs use osmometers to test osmolality of body fluids including but not limited to plasma, serum and urine for diagnostic purposes and for. Osmometry can be expressed in two ways:. Osmolality testing uses state of the art pieces of laboratory instruments known as osmometers. There are two main types, vapor pressure. Osmometry plays a key role in determining molecular. Osmometry Test.
From www.chegg.com
Solved 5. Osmometry (see figure below) is an important Osmometry Test Osmolality testing uses state of the art pieces of laboratory instruments known as osmometers. There are two main types, vapor pressure. Freezing point depression osmometers are utilized to determine a solution's osmotic strength. Osmometry plays a key role in determining molecular weights of solutes based on colligative properties like vapor pressure lowering and. There are two principal methods of osmometry. Osmometry Test.
From www.indiamart.com
Elitech Vapro Vapor Pressure Osmometer for Laboratory, Rs 850000 /piece Osmometry Test Freezing point depression osmometers are utilized to determine a solution's osmotic strength. There are two main types, vapor pressure. An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: Clinical labs. Osmometry Test.
From www.slideserve.com
PPT MLAB 2401 Clinical Chemistry Keri BrophyMartinez PowerPoint Osmometry Test Freezing point depression osmometers are utilized to determine a solution's osmotic strength. Clinical labs use osmometers to test osmolality of body fluids including but not limited to plasma, serum and urine for diagnostic purposes and for. It is the approach that is most. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers:. Osmometry Test.
From www.slideserve.com
PPT Molecular Weight and polymer properties Methods Used to determine Osmometry Test Osmolarity and osmolality are ways to measure and report the osmometry of a solvent. Osmolality testing uses state of the art pieces of laboratory instruments known as osmometers. Osmometry plays a key role in determining molecular weights of solutes based on colligative properties like vapor pressure lowering and. An osmometer is a device used in clinical laboratories for measuring the. Osmometry Test.
From www.reviewofoptometry.com
All About Osmolarity Osmometry Test There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: Osmometry plays a key role in determining molecular weights of solutes based on colligative properties like vapor pressure lowering and. Osmometry can be expressed in two ways:. It is the approach that is most. Freezing point depression osmometers are utilized to determine a. Osmometry Test.
From centralhealthmedical.com
Advanced Osmo1 Osmometer Instruments Osmometry Test There are two main types, vapor pressure. Osmometry plays a key role in determining molecular weights of solutes based on colligative properties like vapor pressure lowering and. An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar. There are two principal methods of osmometry that are suitable for. Osmometry Test.
From syntest.ru
Осмометры Advanced Instruments Представитель в РФ ООО "Синтест Osmometry Test Osmolarity and osmolality are ways to measure and report the osmometry of a solvent. Freezing point depression osmometers are utilized to determine a solution's osmotic strength. It is the approach that is most. An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar. Osmolality testing uses state of. Osmometry Test.
From www.youtube.com
Osmometry Walkthrough YouTube Osmometry Test An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar. Freezing point depression osmometers are utilized to determine a solution's osmotic strength. Osmometry can be expressed in two ways:. Clinical labs use osmometers to test osmolality of body fluids including but not limited to plasma, serum and urine. Osmometry Test.
From centralhealthmedical.com
Advanced Osmo1 Osmometer Instruments Osmometry Test Osmolality testing uses state of the art pieces of laboratory instruments known as osmometers. Osmometry can be expressed in two ways:. It is the approach that is most. Freezing point depression osmometers are utilized to determine a solution's osmotic strength. Osmometry plays a key role in determining molecular weights of solutes based on colligative properties like vapor pressure lowering and.. Osmometry Test.
From www.knauer.net
UHPLC, HPLC, Prep LC, FPLC, SMBC LNP Osmometry KNAUER Osmometry Test Osmolarity and osmolality are ways to measure and report the osmometry of a solvent. There are two main types, vapor pressure. It is the approach that is most. Osmometry plays a key role in determining molecular weights of solutes based on colligative properties like vapor pressure lowering and. There are two principal methods of osmometry that are suitable for determining. Osmometry Test.
From www.purechemistry.org
Osmometry method to determine molecular weight of polymer Purechemistry Osmometry Test Freezing point depression osmometers are utilized to determine a solution's osmotic strength. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: Clinical labs use osmometers to test osmolality of body fluids including but not limited to plasma, serum and urine for diagnostic purposes and for. It is the approach that is most.. Osmometry Test.
From www.scribd.com
osmometry ppt Osmometry Test Freezing point depression osmometers are utilized to determine a solution's osmotic strength. Osmometry can be expressed in two ways:. It is the approach that is most. There are two main types, vapor pressure. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: Clinical labs use osmometers to test osmolality of body fluids. Osmometry Test.
From www.linkedin.com
Osmolality/Osmolarity by vapor pressure osmometry Osmometry Test Osmolarity and osmolality are ways to measure and report the osmometry of a solvent. It is the approach that is most. An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar. Osmometry plays a key role in determining molecular weights of solutes based on colligative properties like vapor. Osmometry Test.
From study.com
Quiz & Worksheet Osmometry Applications Osmometry Test An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: Osmometry plays a key role in determining molecular weights of solutes based on colligative properties like vapor pressure lowering and. Osmometry. Osmometry Test.
From hylandscientific.com
Advanced Instruments Model Osmo1 Osmometer with Accessories Hyland Osmometry Test There are two main types, vapor pressure. Osmolality testing uses state of the art pieces of laboratory instruments known as osmometers. Osmometry plays a key role in determining molecular weights of solutes based on colligative properties like vapor pressure lowering and. Osmolarity and osmolality are ways to measure and report the osmometry of a solvent. It is the approach that. Osmometry Test.
From www.slideshare.net
Osmometry report.pdf Osmometry Test There are two main types, vapor pressure. Freezing point depression osmometers are utilized to determine a solution's osmotic strength. An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar. Osmometry plays a key role in determining molecular weights of solutes based on colligative properties like vapor pressure lowering. Osmometry Test.
From studylib.net
Membrane Osmometry ( , A , χ) M 2 Osmometry Test Osmometry plays a key role in determining molecular weights of solutes based on colligative properties like vapor pressure lowering and. It is the approach that is most. Freezing point depression osmometers are utilized to determine a solution's osmotic strength. There are two main types, vapor pressure. There are two principal methods of osmometry that are suitable for determining average molecular. Osmometry Test.
From www.slideshare.net
Osmometry by Dr. Anurag Yadav Osmometry Test Freezing point depression osmometers are utilized to determine a solution's osmotic strength. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: Clinical labs use osmometers to test osmolality of body fluids including but not limited to plasma, serum and urine for diagnostic purposes and for. An osmometer is a device used in. Osmometry Test.
From slidetodoc.com
Membrane Osmometry Alfredo Clemente CH 392 N Prof Osmometry Test Osmometry plays a key role in determining molecular weights of solutes based on colligative properties like vapor pressure lowering and. Freezing point depression osmometers are utilized to determine a solution's osmotic strength. Clinical labs use osmometers to test osmolality of body fluids including but not limited to plasma, serum and urine for diagnostic purposes and for. Osmolarity and osmolality are. Osmometry Test.
From www.academia.edu
(PDF) Refractometry, test strip, and osmometry compared as measures of Osmometry Test Freezing point depression osmometers are utilized to determine a solution's osmotic strength. Osmometry can be expressed in two ways:. Osmometry plays a key role in determining molecular weights of solutes based on colligative properties like vapor pressure lowering and. Clinical labs use osmometers to test osmolality of body fluids including but not limited to plasma, serum and urine for diagnostic. Osmometry Test.
From www.iconsci.com
What Are Osmometers? Osmometry Test Osmolality testing uses state of the art pieces of laboratory instruments known as osmometers. Osmolarity and osmolality are ways to measure and report the osmometry of a solvent. An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar. It is the approach that is most. Freezing point depression. Osmometry Test.
From ichrom.com
K7400S SemiMicro Osmometer iChrom Solutions Osmometry Test It is the approach that is most. Osmolality testing uses state of the art pieces of laboratory instruments known as osmometers. Osmometry plays a key role in determining molecular weights of solutes based on colligative properties like vapor pressure lowering and. Osmolarity and osmolality are ways to measure and report the osmometry of a solvent. An osmometer is a device. Osmometry Test.
From www.slideshare.net
Osmometry by Dr. Anurag Yadav Osmometry Test Osmolarity and osmolality are ways to measure and report the osmometry of a solvent. Freezing point depression osmometers are utilized to determine a solution's osmotic strength. Osmometry plays a key role in determining molecular weights of solutes based on colligative properties like vapor pressure lowering and. Osmolality testing uses state of the art pieces of laboratory instruments known as osmometers.. Osmometry Test.
From www.slideserve.com
PPT Labs 6 & 7 PowerPoint Presentation, free download ID2180146 Osmometry Test An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar. Osmolality testing uses state of the art pieces of laboratory instruments known as osmometers. There are two main types, vapor pressure. Osmometry plays a key role in determining molecular weights of solutes based on colligative properties like vapor. Osmometry Test.
From www.studocu.com
Osmometry BSc NURSING Studocu Osmometry Test An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar. Clinical labs use osmometers to test osmolality of body fluids including but not limited to plasma, serum and urine for diagnostic purposes and for. Osmometry plays a key role in determining molecular weights of solutes based on colligative. Osmometry Test.
From www.slideshare.net
Osmometry by Dr. Anurag Yadav Osmometry Test Osmometry can be expressed in two ways:. An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar. Osmometry plays a key role in determining molecular weights of solutes based on colligative properties like vapor pressure lowering and. Clinical labs use osmometers to test osmolality of body fluids including. Osmometry Test.
From www.concordscientificdevices.com
vaporpressureosmometer Osmometry Test Osmometry can be expressed in two ways:. Osmometry plays a key role in determining molecular weights of solutes based on colligative properties like vapor pressure lowering and. Osmolarity and osmolality are ways to measure and report the osmometry of a solvent. An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known. Osmometry Test.