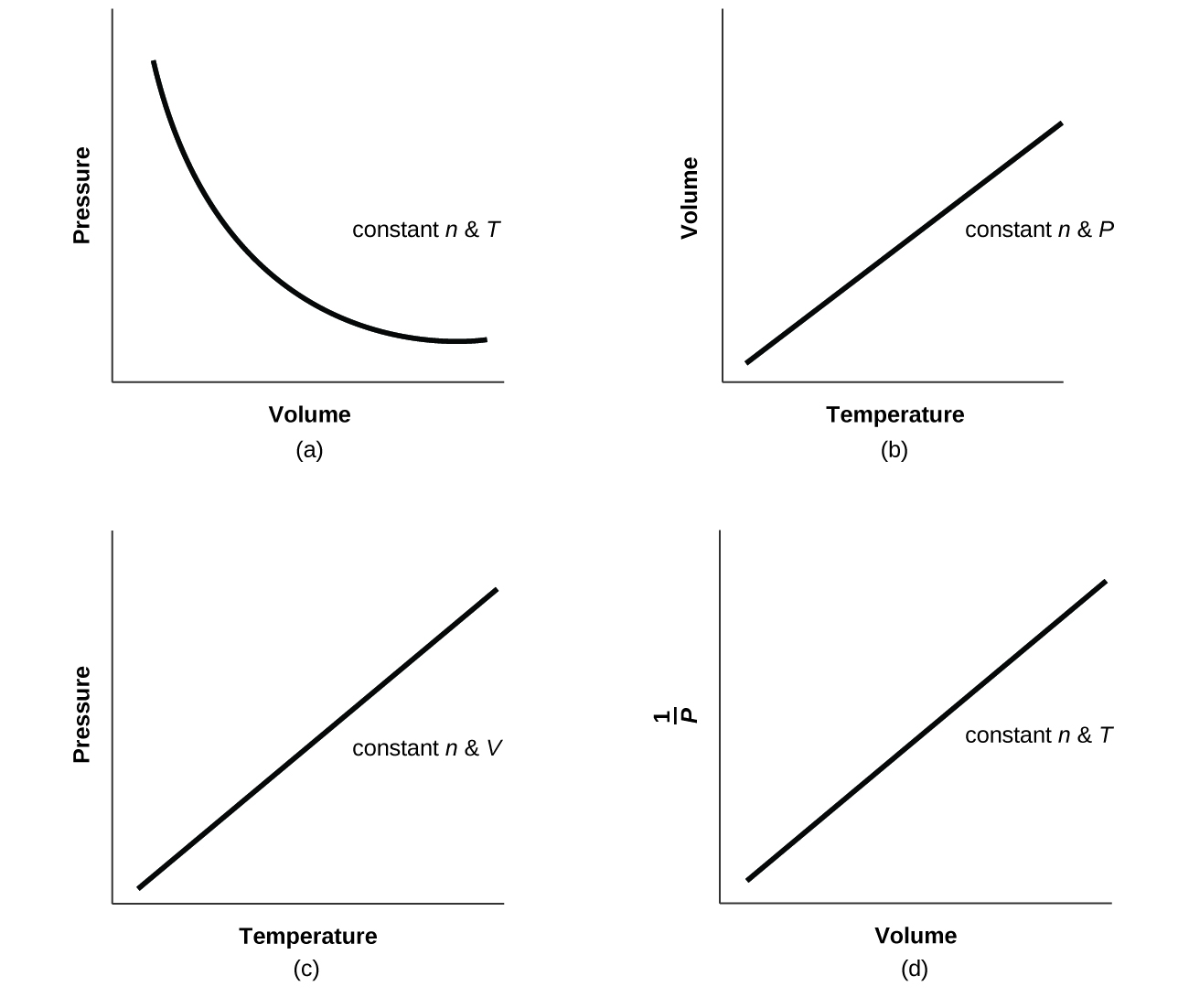
Pressure Absolute Temperature Volume . The ideal gas law can be. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s.
from opentextbc.ca
Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and. The ideal gas law can be. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s.
9.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas
Pressure Absolute Temperature Volume The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The ideal gas law can be. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and.
From www.numerade.com
SOLVED Label each I variable in the equation belowwilh (he property it Pressure Absolute Temperature Volume The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and. The pressure of a given amount. Pressure Absolute Temperature Volume.
From www.meritnation.com
if the pressure and absolute temperature of 2 litres of co2 are doubled Pressure Absolute Temperature Volume Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and. The ideal gas law can be. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s.. Pressure Absolute Temperature Volume.
From www.chegg.com
Solved Which of the following will cause the volume of an Pressure Absolute Temperature Volume The ideal gas law can be. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. Early scientists explored the relationships among the pressure. Pressure Absolute Temperature Volume.
From www.numerade.com
SOLVED What happens to the temperature of a confined gas if the Pressure Absolute Temperature Volume The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The ideal gas law can be. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount of gas. Pressure Absolute Temperature Volume.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Pressure Absolute Temperature Volume The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount of gas is directly proportional to its absolute. Pressure Absolute Temperature Volume.
From owlcation.com
The Theories and Behavior of Gas Owlcation Pressure Absolute Temperature Volume The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and. The pressure of a given amount. Pressure Absolute Temperature Volume.
From stock.adobe.com
Vektorová grafika „Gay lussac law infographic diagram example ideal gas Pressure Absolute Temperature Volume The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The ideal gas law can be. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount of gas. Pressure Absolute Temperature Volume.
From www.slideserve.com
PPT Chapter 10 Gases & the Atmosphere PowerPoint Presentation ID611285 Pressure Absolute Temperature Volume The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and. The pressure of a given amount. Pressure Absolute Temperature Volume.
From www.slideserve.com
PPT Unit 5 Gases More Gas Laws Charles’s Law and Boyle’s Law Pressure Absolute Temperature Volume The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The ideal gas law can be. The pressure of a given amount of gas. Pressure Absolute Temperature Volume.
From sciencenotes.org
What Is Absolute Temperature? Definition and Scales Pressure Absolute Temperature Volume Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and. The ideal gas law can be. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s.. Pressure Absolute Temperature Volume.
From chem.libretexts.org
6.3 Relationships among Pressure, Temperature, Volume, and Amount Pressure Absolute Temperature Volume The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount of gas is directly proportional to its absolute. Pressure Absolute Temperature Volume.
From www.numerade.com
SOLVED Which of the following graphs shows the correct relationship Pressure Absolute Temperature Volume The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and. The pressure of a given amount. Pressure Absolute Temperature Volume.
From www.numerade.com
A volume of 450 cm3 of air is measured at a pressure of 740 mm Hg Pressure Absolute Temperature Volume The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The ideal gas law can be. The pressure of a given amount of gas. Pressure Absolute Temperature Volume.
From www.toppr.com
22. 22. At a constant pressure, of the following graphs the one which Pressure Absolute Temperature Volume The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The ideal gas law can be. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount of gas. Pressure Absolute Temperature Volume.
From cartoondealer.com
Boyle's Law, Relationship Between Pressure And Volume Of Gas At Pressure Absolute Temperature Volume The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The ideal gas law can be. The pressure of a given amount of gas. Pressure Absolute Temperature Volume.
From tommyfersdouglas.blogspot.com
Relationship Between Pressure and Temperature Pressure Absolute Temperature Volume The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The ideal gas law can be. Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and.. Pressure Absolute Temperature Volume.
From www.britannica.com
Pressure Definition, Measurement, & Types Britannica Pressure Absolute Temperature Volume Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount. Pressure Absolute Temperature Volume.
From www.alamy.com
Charles's law. relationship between volume and temperature. Increasing Pressure Absolute Temperature Volume Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount. Pressure Absolute Temperature Volume.
From www.dreamstime.com
Ideal Gas Law. Boyles Law Pressure Volume Relationship in Gases Stock Pressure Absolute Temperature Volume Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The ideal gas law can be.. Pressure Absolute Temperature Volume.
From www.pinterest.co.uk
A mathematical derivation of the equations relating the pressure Pressure Absolute Temperature Volume The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. Early scientists explored the relationships among the pressure of a gas (p) and its. Pressure Absolute Temperature Volume.
From www.youtube.com
Pressure, Volume, Temperature and Mole Relationships YouTube Pressure Absolute Temperature Volume The ideal gas law can be. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount of gas. Pressure Absolute Temperature Volume.
From www.coursehero.com
[Solved] Need help with this one Question 2 At constant pressure, which Pressure Absolute Temperature Volume The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and. The pressure of a given amount. Pressure Absolute Temperature Volume.
From www.youtube.com
1.4.6 Solve problems involving temperature, pressure and volume for an Pressure Absolute Temperature Volume The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The ideal gas law can be. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount of gas. Pressure Absolute Temperature Volume.
From flatworldknowledge.lardbucket.org
Relationships among Pressure, Temperature, Volume, and Amount Pressure Absolute Temperature Volume The ideal gas law can be. Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s.. Pressure Absolute Temperature Volume.
From chem.libretexts.org
6.3 Relationships among Pressure, Temperature, Volume, and Amount Pressure Absolute Temperature Volume The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. Early scientists explored the relationships among the pressure of a gas (p) and its. Pressure Absolute Temperature Volume.
From www.toppr.com
Two containers of equal volume contain the same gasat pressures P 1 and Pressure Absolute Temperature Volume The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The ideal gas law can be. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount of gas. Pressure Absolute Temperature Volume.
From www.toppr.com
A sample of an ideal gas occupies a volume V at a pressure P and Pressure Absolute Temperature Volume The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount of gas is directly proportional to its absolute. Pressure Absolute Temperature Volume.
From www.slideserve.com
PPT CHAPTER 12 GASES AND THEORY PowerPoint Pressure Absolute Temperature Volume The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. Early scientists explored the relationships among the pressure of a gas (p) and its. Pressure Absolute Temperature Volume.
From www.dreamstime.com
Boyle S Law Showing the Pressure and Volume Relationship Stock Vector Pressure Absolute Temperature Volume The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The ideal gas law can be. Early scientists explored the relationships among the pressure. Pressure Absolute Temperature Volume.
From opentextbc.ca
9.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Pressure Absolute Temperature Volume The ideal gas law can be. Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s.. Pressure Absolute Temperature Volume.
From opentextbc.ca
9.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Pressure Absolute Temperature Volume Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The ideal gas law can be.. Pressure Absolute Temperature Volume.
From socratic.org
Which graph shows the relationship between the temperature and volume Pressure Absolute Temperature Volume Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount. Pressure Absolute Temperature Volume.
From www.youtube.com
Pressure, Volume and Temperature Relationships Chemistry Tutorial Pressure Absolute Temperature Volume The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and. The pressure of a given amount. Pressure Absolute Temperature Volume.
From brainly.com
Which graph shows the pressuretemperature relationship for a gas at a Pressure Absolute Temperature Volume Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount. Pressure Absolute Temperature Volume.
From www.youtube.com
If the absolute temperature of a gas is doubled and the pressure is Pressure Absolute Temperature Volume The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The ideal gas law can be. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s. The pressure of a given amount of gas. Pressure Absolute Temperature Volume.