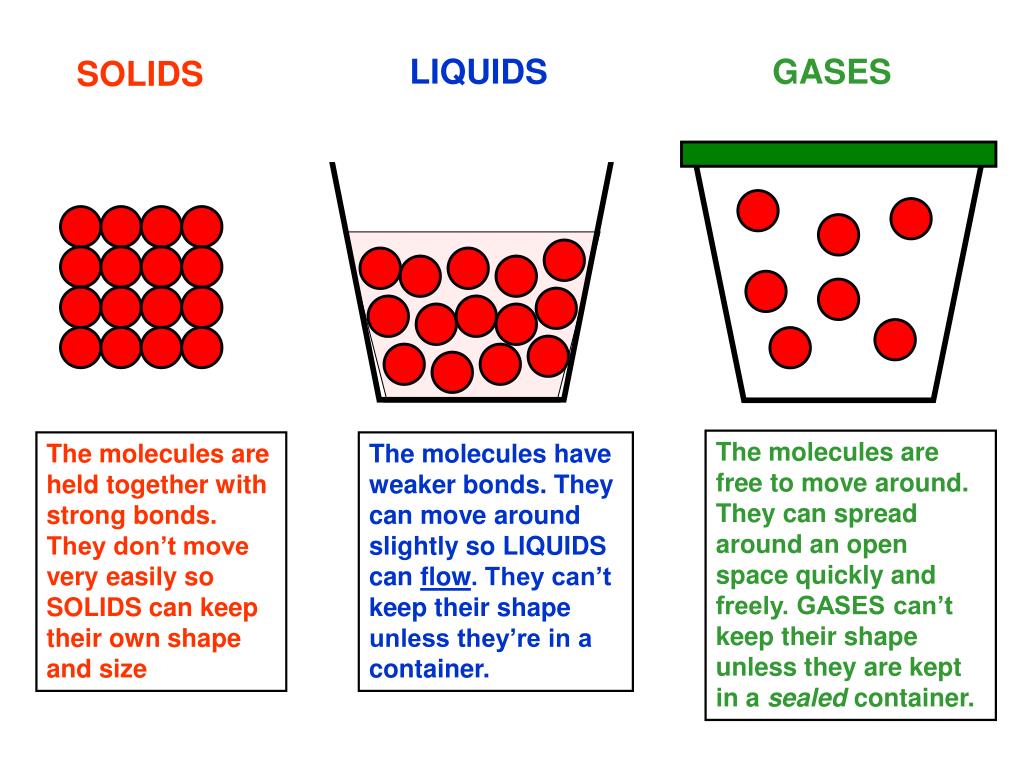
Solid Back Into Liquid . As we keep heating, the. Every element and substance can transition from one phase to another at a. After gaining sufficient energy, the molecules of ice escape out of the ice cube and into the beaker and in doing so undergo a change in state from solid to liquid. Scientists use something called a freezing point or melting point to measure the temperature at which a liquid turns into a solid. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a. There are physical effects that can change the melting point. Processes such as evaporation and boiling change the state of. Solids, liquids and gases change state when they are heated or cooled. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. The liquid must first be cooled to its freezing point (the same temperature as its melting.
from www.slideserve.com
Every element and substance can transition from one phase to another at a. As we keep heating, the. The liquid must first be cooled to its freezing point (the same temperature as its melting. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Scientists use something called a freezing point or melting point to measure the temperature at which a liquid turns into a solid. There are physical effects that can change the melting point. Solids, liquids and gases change state when they are heated or cooled. After gaining sufficient energy, the molecules of ice escape out of the ice cube and into the beaker and in doing so undergo a change in state from solid to liquid. Processes such as evaporation and boiling change the state of. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a.
PPT SOLIDS LIQUIDS GASES PowerPoint Presentation, free download ID
Solid Back Into Liquid Processes such as evaporation and boiling change the state of. There are physical effects that can change the melting point. After gaining sufficient energy, the molecules of ice escape out of the ice cube and into the beaker and in doing so undergo a change in state from solid to liquid. Solids, liquids and gases change state when they are heated or cooled. Every element and substance can transition from one phase to another at a. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a. Processes such as evaporation and boiling change the state of. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Scientists use something called a freezing point or melting point to measure the temperature at which a liquid turns into a solid. The liquid must first be cooled to its freezing point (the same temperature as its melting. As we keep heating, the.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Solid Back Into Liquid Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Scientists use something called a freezing point or melting point to measure the temperature at which a liquid turns into a solid. As we keep heating, the. Solids, liquids and gases change state when they are heated or cooled. After gaining sufficient. Solid Back Into Liquid.
From itinerantmission.blogspot.com
Itinerant Mission 3 Physical States of Matter Solid Liquid Gas Solid Back Into Liquid We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a. Solids, liquids and gases change state when they are heated or cooled. Scientists use something called a freezing point or melting point to measure the. Solid Back Into Liquid.
From www.snexplores.org
Explainer What are the different states of matter? Solid Back Into Liquid Scientists use something called a freezing point or melting point to measure the temperature at which a liquid turns into a solid. After gaining sufficient energy, the molecules of ice escape out of the ice cube and into the beaker and in doing so undergo a change in state from solid to liquid. Phase transition is when a substance changes. Solid Back Into Liquid.
From mungfali.com
Solid Liquid Gas Anchor Chart Solid Back Into Liquid The liquid must first be cooled to its freezing point (the same temperature as its melting. Scientists use something called a freezing point or melting point to measure the temperature at which a liquid turns into a solid. Every element and substance can transition from one phase to another at a. Processes such as evaporation and boiling change the state. Solid Back Into Liquid.
From earthhow.com
States of Water Gas, Liquid and Solid Earth How Solid Back Into Liquid Scientists use something called a freezing point or melting point to measure the temperature at which a liquid turns into a solid. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a. Processes such as. Solid Back Into Liquid.
From exoxqcgtx.blob.core.windows.net
Solid Into Liquid Is at Alisa McGowan blog Solid Back Into Liquid The liquid must first be cooled to its freezing point (the same temperature as its melting. Scientists use something called a freezing point or melting point to measure the temperature at which a liquid turns into a solid. Solids, liquids and gases change state when they are heated or cooled. Processes such as evaporation and boiling change the state of.. Solid Back Into Liquid.
From www.vedantu.com
Which Liquid Solid on Heating Learn Important Terms and Concepts Solid Back Into Liquid Every element and substance can transition from one phase to another at a. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a. As we keep heating, the. The liquid must first be cooled to. Solid Back Into Liquid.
From byjus.com
What is the name of the process when a liquid changes into a gas? Solid Back Into Liquid After gaining sufficient energy, the molecules of ice escape out of the ice cube and into the beaker and in doing so undergo a change in state from solid to liquid. Scientists use something called a freezing point or melting point to measure the temperature at which a liquid turns into a solid. Phase transition is when a substance changes. Solid Back Into Liquid.
From www.wikihow.com
How to Make a Liquid into a Solid 4 Fun Science Experiments Solid Back Into Liquid After gaining sufficient energy, the molecules of ice escape out of the ice cube and into the beaker and in doing so undergo a change in state from solid to liquid. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to. Solid Back Into Liquid.
From exoxqcgtx.blob.core.windows.net
Solid Into Liquid Is at Alisa McGowan blog Solid Back Into Liquid We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a. Scientists use something called a freezing point or melting point to measure the temperature at which a liquid turns into a solid. Every element and. Solid Back Into Liquid.
From saylordotorg.github.io
Solids and Liquids Solid Back Into Liquid Solids, liquids and gases change state when they are heated or cooled. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a. Phase transition is when a substance changes from a solid, liquid, or gas. Solid Back Into Liquid.
From www.baamboozle.com
Matter and Changes in Liquid Baamboozle Baamboozle The Most Fun Solid Back Into Liquid We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a. Processes such as evaporation and boiling change the state of. Scientists use something called a freezing point or melting point to measure the temperature at. Solid Back Into Liquid.
From www.ase.org.uk
Solids, liquids and gases Solid Back Into Liquid We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a. Solids, liquids and gases change state when they are heated or cooled. Processes such as evaporation and boiling change the state of. Every element and. Solid Back Into Liquid.
From sebschemistry.blogspot.com
IGCSE Edexcel Chemistry Help 1.1 understand the arrangement, movement Solid Back Into Liquid We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a. Every element and substance can transition from one phase to another at a. As we keep heating, the. After gaining sufficient energy, the molecules of. Solid Back Into Liquid.
From primaryleap.co.uk
Chemistry States Of Matter Level 2 activity for kids PrimaryLeap.co.uk Solid Back Into Liquid There are physical effects that can change the melting point. Solids, liquids and gases change state when they are heated or cooled. Processes such as evaporation and boiling change the state of. As we keep heating, the. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool. Solid Back Into Liquid.
From byjus.com
Perform an activity to find out how to dissolve a solid in a liquid? Solid Back Into Liquid After gaining sufficient energy, the molecules of ice escape out of the ice cube and into the beaker and in doing so undergo a change in state from solid to liquid. Solids, liquids and gases change state when they are heated or cooled. We take advantage of changes between the gas, liquid, and solid states to cool a drink with. Solid Back Into Liquid.
From exoxqcgtx.blob.core.windows.net
Solid Into Liquid Is at Alisa McGowan blog Solid Back Into Liquid Processes such as evaporation and boiling change the state of. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a. There are physical effects that can change the melting point. Every element and substance can. Solid Back Into Liquid.
From www.vecteezy.com
Changing the state of matter from solid, liquid and gas due to Solid Back Into Liquid Solids, liquids and gases change state when they are heated or cooled. As we keep heating, the. Processes such as evaporation and boiling change the state of. The liquid must first be cooled to its freezing point (the same temperature as its melting. There are physical effects that can change the melting point. Scientists use something called a freezing point. Solid Back Into Liquid.
From mainanspektakuler.blogspot.com
25+ Gambar Mencair Solid Back Into Liquid Scientists use something called a freezing point or melting point to measure the temperature at which a liquid turns into a solid. There are physical effects that can change the melting point. Every element and substance can transition from one phase to another at a. The liquid must first be cooled to its freezing point (the same temperature as its. Solid Back Into Liquid.
From www.naturphilosophie.co.uk
Changing States Fundamental Phases of Matter NaturPhilosophie Solid Back Into Liquid Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. As we keep heating, the. Solids, liquids and gases change state when they are heated or cooled. The liquid must first be cooled to its freezing point (the same temperature as its melting. Scientists use something called a freezing point or melting. Solid Back Into Liquid.
From www.wikihow.com
4 Ways to Make a Liquid Into a Solid wikiHow Solid Back Into Liquid Solids, liquids and gases change state when they are heated or cooled. Every element and substance can transition from one phase to another at a. As we keep heating, the. After gaining sufficient energy, the molecules of ice escape out of the ice cube and into the beaker and in doing so undergo a change in state from solid to. Solid Back Into Liquid.
From www.youtube.com
Experiment (turning liquid milk into a solid) YouTube Solid Back Into Liquid After gaining sufficient energy, the molecules of ice escape out of the ice cube and into the beaker and in doing so undergo a change in state from solid to liquid. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to. Solid Back Into Liquid.
From www.vecteezy.com
Solid Liquid Gas Vector Art, Icons, and Graphics for Free Download Solid Back Into Liquid Processes such as evaporation and boiling change the state of. As we keep heating, the. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a. The liquid must first be cooled to its freezing point. Solid Back Into Liquid.
From www.slideserve.com
PPT SOLIDS LIQUIDS GASES PowerPoint Presentation, free download ID Solid Back Into Liquid Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. There are physical effects that can change the melting point. Processes such as evaporation and boiling change the state of. Scientists use something called a freezing point or melting point to measure the temperature at which a liquid turns into a solid.. Solid Back Into Liquid.
From ar.inspiredpencil.com
Science Liquids Solids And Gases Solid Back Into Liquid Scientists use something called a freezing point or melting point to measure the temperature at which a liquid turns into a solid. After gaining sufficient energy, the molecules of ice escape out of the ice cube and into the beaker and in doing so undergo a change in state from solid to liquid. Every element and substance can transition from. Solid Back Into Liquid.
From shaunmwilliams.com
Lecture 1 Presentation Solid Back Into Liquid We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a. Solids, liquids and gases change state when they are heated or cooled. Phase transition is when a substance changes from a solid, liquid, or gas. Solid Back Into Liquid.
From www.dreamstime.com
Solution. Solid in liquid stock vector. Illustration of expansion Solid Back Into Liquid After gaining sufficient energy, the molecules of ice escape out of the ice cube and into the beaker and in doing so undergo a change in state from solid to liquid. The liquid must first be cooled to its freezing point (the same temperature as its melting. Processes such as evaporation and boiling change the state of. There are physical. Solid Back Into Liquid.
From www.youtube.com
Science Lesson 4 Mixing Solids into Liquids YouTube Solid Back Into Liquid Scientists use something called a freezing point or melting point to measure the temperature at which a liquid turns into a solid. As we keep heating, the. There are physical effects that can change the melting point. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool. Solid Back Into Liquid.
From www.teachoo.com
Diffusion in Solids, Liquids and Gases with Examples Teachoo Solid Back Into Liquid As we keep heating, the. Scientists use something called a freezing point or melting point to measure the temperature at which a liquid turns into a solid. There are physical effects that can change the melting point. Every element and substance can transition from one phase to another at a. Phase transition is when a substance changes from a solid,. Solid Back Into Liquid.
From www.geeksforgeeks.org
Difference Between Solid, Liquid, and Gas In Tabular Form Solid Back Into Liquid Scientists use something called a freezing point or melting point to measure the temperature at which a liquid turns into a solid. There are physical effects that can change the melting point. Solids, liquids and gases change state when they are heated or cooled. Phase transition is when a substance changes from a solid, liquid, or gas state to a. Solid Back Into Liquid.
From wirelistlatinised.z21.web.core.windows.net
Solid Liquid Gas Diagram Solid Back Into Liquid Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a. Scientists use something called a freezing point. Solid Back Into Liquid.
From animalia-life.club
Solid Examples For Kids Solid Back Into Liquid As we keep heating, the. Solids, liquids and gases change state when they are heated or cooled. Every element and substance can transition from one phase to another at a. There are physical effects that can change the melting point. Processes such as evaporation and boiling change the state of. We take advantage of changes between the gas, liquid, and. Solid Back Into Liquid.
From brainly.in
11. Which of the following processes is known as(a) Change of liquid to Solid Back Into Liquid Every element and substance can transition from one phase to another at a. Scientists use something called a freezing point or melting point to measure the temperature at which a liquid turns into a solid. Solids, liquids and gases change state when they are heated or cooled. After gaining sufficient energy, the molecules of ice escape out of the ice. Solid Back Into Liquid.
From johnwest.edublogs.org
Solids liquids and gasses, a story in three parts. Science Connected Solid Back Into Liquid After gaining sufficient energy, the molecules of ice escape out of the ice cube and into the beaker and in doing so undergo a change in state from solid to liquid. Solids, liquids and gases change state when they are heated or cooled. Phase transition is when a substance changes from a solid, liquid, or gas state to a different. Solid Back Into Liquid.
From www.scribd.com
Solid Into Liquid PDF Solid Back Into Liquid As we keep heating, the. Scientists use something called a freezing point or melting point to measure the temperature at which a liquid turns into a solid. Processes such as evaporation and boiling change the state of. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool. Solid Back Into Liquid.