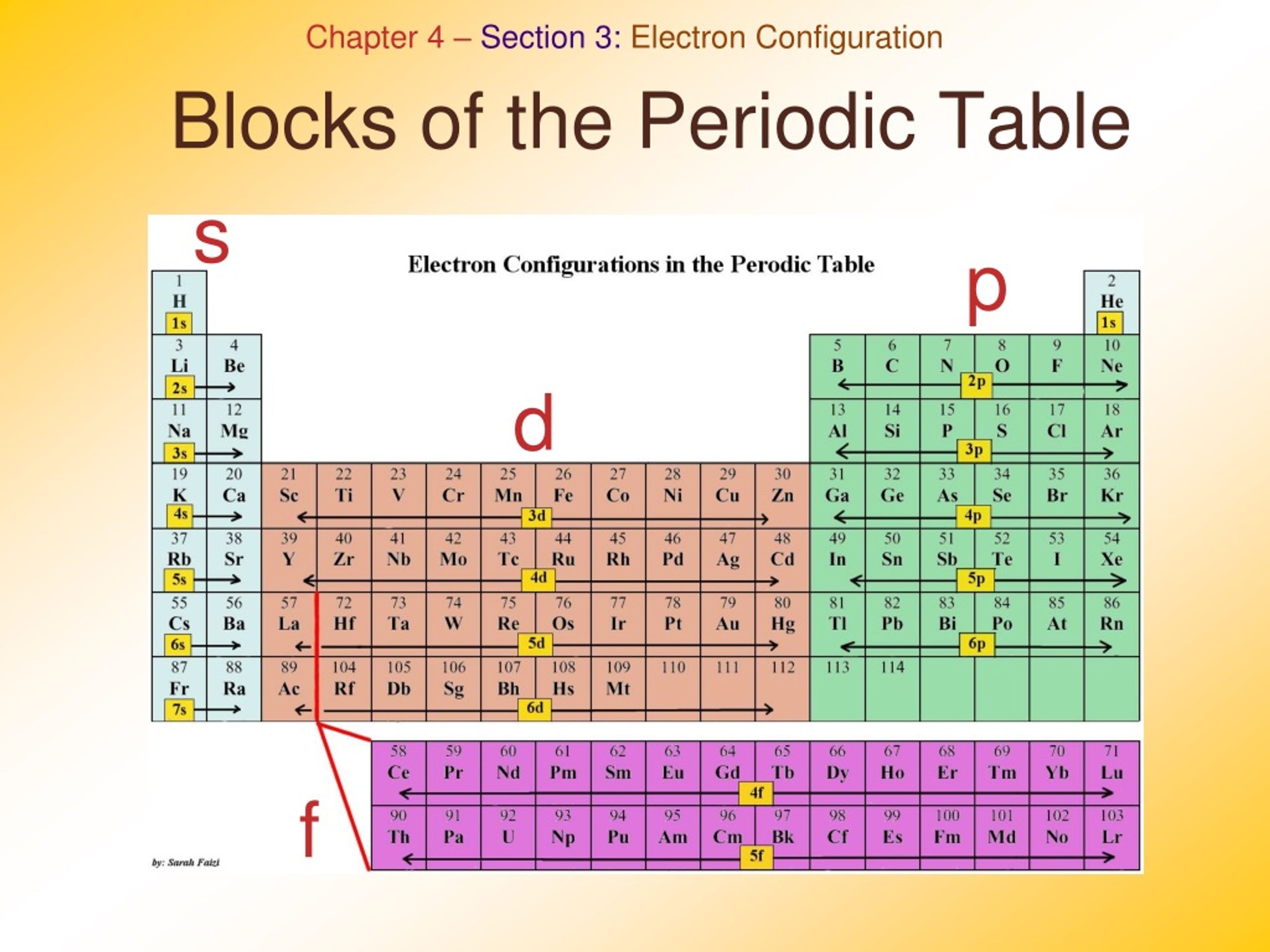
D Block Periodic Table Definition . Transition elements are those elements that have partially or incompletely filled d orbital in their ground state or the most stable oxidation state. Their general electron configuration is [noblegas] (n −1) d1−10ns0−2. This is why they are called “transition” metals. The “d” stands for “diffuse” and the azimuthal quantum number is 2. These elements are all metals, usually with two or more oxidation states. They are called so because the highest energy subshell, i.e., the subshell where the last electron occupies, is a d subshell What are d block elements in chemistry?
from www.slideserve.com
These elements are all metals, usually with two or more oxidation states. They are called so because the highest energy subshell, i.e., the subshell where the last electron occupies, is a d subshell Transition elements are those elements that have partially or incompletely filled d orbital in their ground state or the most stable oxidation state. This is why they are called “transition” metals. Their general electron configuration is [noblegas] (n −1) d1−10ns0−2. What are d block elements in chemistry? The “d” stands for “diffuse” and the azimuthal quantum number is 2.
PPT Unit 2 PowerPoint Presentation, free download ID298336
D Block Periodic Table Definition What are d block elements in chemistry? These elements are all metals, usually with two or more oxidation states. This is why they are called “transition” metals. Transition elements are those elements that have partially or incompletely filled d orbital in their ground state or the most stable oxidation state. The “d” stands for “diffuse” and the azimuthal quantum number is 2. They are called so because the highest energy subshell, i.e., the subshell where the last electron occupies, is a d subshell What are d block elements in chemistry? Their general electron configuration is [noblegas] (n −1) d1−10ns0−2.
From inspiritvr.com
Blocks in periodic table Study Guide Inspirit D Block Periodic Table Definition Their general electron configuration is [noblegas] (n −1) d1−10ns0−2. This is why they are called “transition” metals. Transition elements are those elements that have partially or incompletely filled d orbital in their ground state or the most stable oxidation state. What are d block elements in chemistry? They are called so because the highest energy subshell, i.e., the subshell where. D Block Periodic Table Definition.
From chemistnotes.com
d block elements, name list, useful properties Chemistry Notes D Block Periodic Table Definition They are called so because the highest energy subshell, i.e., the subshell where the last electron occupies, is a d subshell The “d” stands for “diffuse” and the azimuthal quantum number is 2. Transition elements are those elements that have partially or incompletely filled d orbital in their ground state or the most stable oxidation state. These elements are all. D Block Periodic Table Definition.
From studigoo.com
P Block elements notes for Neet StudiGoo D Block Periodic Table Definition This is why they are called “transition” metals. They are called so because the highest energy subshell, i.e., the subshell where the last electron occupies, is a d subshell Transition elements are those elements that have partially or incompletely filled d orbital in their ground state or the most stable oxidation state. These elements are all metals, usually with two. D Block Periodic Table Definition.
From www.chemistrylearner.com
dBlock Elements Definition and Characteristics D Block Periodic Table Definition These elements are all metals, usually with two or more oxidation states. What are d block elements in chemistry? They are called so because the highest energy subshell, i.e., the subshell where the last electron occupies, is a d subshell Their general electron configuration is [noblegas] (n −1) d1−10ns0−2. This is why they are called “transition” metals. The “d” stands. D Block Periodic Table Definition.
From mungfali.com
Periodic Table Of Elements With Orbitals D Block Periodic Table Definition They are called so because the highest energy subshell, i.e., the subshell where the last electron occupies, is a d subshell What are d block elements in chemistry? These elements are all metals, usually with two or more oxidation states. The “d” stands for “diffuse” and the azimuthal quantum number is 2. This is why they are called “transition” metals.. D Block Periodic Table Definition.
From mavink.com
Periodic Table Split Into Blocks D Block Periodic Table Definition What are d block elements in chemistry? They are called so because the highest energy subshell, i.e., the subshell where the last electron occupies, is a d subshell Their general electron configuration is [noblegas] (n −1) d1−10ns0−2. These elements are all metals, usually with two or more oxidation states. The “d” stands for “diffuse” and the azimuthal quantum number is. D Block Periodic Table Definition.
From testbook.com
Blocks of the Periodic Table sblock, pblock, dblock, fblock D Block Periodic Table Definition These elements are all metals, usually with two or more oxidation states. The “d” stands for “diffuse” and the azimuthal quantum number is 2. They are called so because the highest energy subshell, i.e., the subshell where the last electron occupies, is a d subshell Transition elements are those elements that have partially or incompletely filled d orbital in their. D Block Periodic Table Definition.
From cabinet.matttroy.net
Periodic Table D Block Elements Names Matttroy D Block Periodic Table Definition These elements are all metals, usually with two or more oxidation states. This is why they are called “transition” metals. Transition elements are those elements that have partially or incompletely filled d orbital in their ground state or the most stable oxidation state. They are called so because the highest energy subshell, i.e., the subshell where the last electron occupies,. D Block Periodic Table Definition.
From www.alamy.com
Periodic table of the elements colored according to their block s, p D Block Periodic Table Definition What are d block elements in chemistry? Their general electron configuration is [noblegas] (n −1) d1−10ns0−2. The “d” stands for “diffuse” and the azimuthal quantum number is 2. Transition elements are those elements that have partially or incompletely filled d orbital in their ground state or the most stable oxidation state. They are called so because the highest energy subshell,. D Block Periodic Table Definition.
From scienceline.org
The FBlock An introduction Scienceline D Block Periodic Table Definition The “d” stands for “diffuse” and the azimuthal quantum number is 2. Their general electron configuration is [noblegas] (n −1) d1−10ns0−2. What are d block elements in chemistry? These elements are all metals, usually with two or more oxidation states. They are called so because the highest energy subshell, i.e., the subshell where the last electron occupies, is a d. D Block Periodic Table Definition.
From www.pw.live
D Block Elements Notes, D Block Elements In Periodic Table, D Block D Block Periodic Table Definition Their general electron configuration is [noblegas] (n −1) d1−10ns0−2. What are d block elements in chemistry? Transition elements are those elements that have partially or incompletely filled d orbital in their ground state or the most stable oxidation state. These elements are all metals, usually with two or more oxidation states. They are called so because the highest energy subshell,. D Block Periodic Table Definition.
From www.britannica.com
transition metal Definition, Properties, Elements, & Facts Britannica D Block Periodic Table Definition What are d block elements in chemistry? The “d” stands for “diffuse” and the azimuthal quantum number is 2. They are called so because the highest energy subshell, i.e., the subshell where the last electron occupies, is a d subshell Their general electron configuration is [noblegas] (n −1) d1−10ns0−2. This is why they are called “transition” metals. These elements are. D Block Periodic Table Definition.
From mungfali.com
Periodic Table D Block Elements D Block Periodic Table Definition The “d” stands for “diffuse” and the azimuthal quantum number is 2. These elements are all metals, usually with two or more oxidation states. What are d block elements in chemistry? Transition elements are those elements that have partially or incompletely filled d orbital in their ground state or the most stable oxidation state. They are called so because the. D Block Periodic Table Definition.
From www.shutterstock.com
48 D Block In Periodic Table Images, Stock Photos & Vectors Shutterstock D Block Periodic Table Definition They are called so because the highest energy subshell, i.e., the subshell where the last electron occupies, is a d subshell What are d block elements in chemistry? This is why they are called “transition” metals. The “d” stands for “diffuse” and the azimuthal quantum number is 2. Their general electron configuration is [noblegas] (n −1) d1−10ns0−2. Transition elements are. D Block Periodic Table Definition.
From infinitylearn.com
D Block Elements Infinity Learn by Sri Chaitanya D Block Periodic Table Definition These elements are all metals, usually with two or more oxidation states. Their general electron configuration is [noblegas] (n −1) d1−10ns0−2. This is why they are called “transition” metals. What are d block elements in chemistry? They are called so because the highest energy subshell, i.e., the subshell where the last electron occupies, is a d subshell The “d” stands. D Block Periodic Table Definition.
From app.jove.com
Transition Metals Electron Configurations and Properties Concept D Block Periodic Table Definition What are d block elements in chemistry? This is why they are called “transition” metals. These elements are all metals, usually with two or more oxidation states. Their general electron configuration is [noblegas] (n −1) d1−10ns0−2. The “d” stands for “diffuse” and the azimuthal quantum number is 2. Transition elements are those elements that have partially or incompletely filled d. D Block Periodic Table Definition.
From www.slideserve.com
PPT Unit 2 PowerPoint Presentation, free download ID298336 D Block Periodic Table Definition What are d block elements in chemistry? The “d” stands for “diffuse” and the azimuthal quantum number is 2. This is why they are called “transition” metals. They are called so because the highest energy subshell, i.e., the subshell where the last electron occupies, is a d subshell Transition elements are those elements that have partially or incompletely filled d. D Block Periodic Table Definition.
From pediaa.com
Difference Between d and f Block Elements Definition, Properties D Block Periodic Table Definition What are d block elements in chemistry? Transition elements are those elements that have partially or incompletely filled d orbital in their ground state or the most stable oxidation state. This is why they are called “transition” metals. These elements are all metals, usually with two or more oxidation states. The “d” stands for “diffuse” and the azimuthal quantum number. D Block Periodic Table Definition.
From cabinet.matttroy.net
Periodic Table D Block Elements Names Matttroy D Block Periodic Table Definition Their general electron configuration is [noblegas] (n −1) d1−10ns0−2. What are d block elements in chemistry? They are called so because the highest energy subshell, i.e., the subshell where the last electron occupies, is a d subshell This is why they are called “transition” metals. These elements are all metals, usually with two or more oxidation states. The “d” stands. D Block Periodic Table Definition.
From cabinet.matttroy.net
In Periodic Table D Block Matttroy D Block Periodic Table Definition What are d block elements in chemistry? They are called so because the highest energy subshell, i.e., the subshell where the last electron occupies, is a d subshell Their general electron configuration is [noblegas] (n −1) d1−10ns0−2. This is why they are called “transition” metals. These elements are all metals, usually with two or more oxidation states. Transition elements are. D Block Periodic Table Definition.
From www.linstitute.net
IB DP Chemistry HL复习笔记3.1.1 The Periodic Table翰林国际教育 D Block Periodic Table Definition This is why they are called “transition” metals. Their general electron configuration is [noblegas] (n −1) d1−10ns0−2. What are d block elements in chemistry? Transition elements are those elements that have partially or incompletely filled d orbital in their ground state or the most stable oxidation state. They are called so because the highest energy subshell, i.e., the subshell where. D Block Periodic Table Definition.
From worksheetsday.blogspot.com
Periodic Table Hd Images Black And White Worksheets D Block Periodic Table Definition The “d” stands for “diffuse” and the azimuthal quantum number is 2. These elements are all metals, usually with two or more oxidation states. Their general electron configuration is [noblegas] (n −1) d1−10ns0−2. They are called so because the highest energy subshell, i.e., the subshell where the last electron occupies, is a d subshell Transition elements are those elements that. D Block Periodic Table Definition.
From pediaa.com
What is the Difference Between PBlock and DBlock Elements D Block Periodic Table Definition Their general electron configuration is [noblegas] (n −1) d1−10ns0−2. What are d block elements in chemistry? This is why they are called “transition” metals. The “d” stands for “diffuse” and the azimuthal quantum number is 2. Transition elements are those elements that have partially or incompletely filled d orbital in their ground state or the most stable oxidation state. They. D Block Periodic Table Definition.
From www.neetexambooster.in
d and f block elements important points NCERT Chemistry Class 12 D Block Periodic Table Definition The “d” stands for “diffuse” and the azimuthal quantum number is 2. They are called so because the highest energy subshell, i.e., the subshell where the last electron occupies, is a d subshell Their general electron configuration is [noblegas] (n −1) d1−10ns0−2. What are d block elements in chemistry? Transition elements are those elements that have partially or incompletely filled. D Block Periodic Table Definition.
From cabinet.matttroy.net
Periodic Table D Block Elements Names Matttroy D Block Periodic Table Definition What are d block elements in chemistry? This is why they are called “transition” metals. Their general electron configuration is [noblegas] (n −1) d1−10ns0−2. The “d” stands for “diffuse” and the azimuthal quantum number is 2. These elements are all metals, usually with two or more oxidation states. Transition elements are those elements that have partially or incompletely filled d. D Block Periodic Table Definition.
From www.ck12.org
A block diagram of the periodic table shows which sublevels are being D Block Periodic Table Definition These elements are all metals, usually with two or more oxidation states. The “d” stands for “diffuse” and the azimuthal quantum number is 2. This is why they are called “transition” metals. They are called so because the highest energy subshell, i.e., the subshell where the last electron occupies, is a d subshell What are d block elements in chemistry?. D Block Periodic Table Definition.
From tableformeonly.blogspot.com
What Are The Transition Metals On The Periodic Table D Block Periodic Table Definition Their general electron configuration is [noblegas] (n −1) d1−10ns0−2. Transition elements are those elements that have partially or incompletely filled d orbital in their ground state or the most stable oxidation state. What are d block elements in chemistry? The “d” stands for “diffuse” and the azimuthal quantum number is 2. These elements are all metals, usually with two or. D Block Periodic Table Definition.
From blog.askiitians.com
These Daily Uses of pblock Elements will Surprise You askIITians D Block Periodic Table Definition They are called so because the highest energy subshell, i.e., the subshell where the last electron occupies, is a d subshell Transition elements are those elements that have partially or incompletely filled d orbital in their ground state or the most stable oxidation state. Their general electron configuration is [noblegas] (n −1) d1−10ns0−2. What are d block elements in chemistry?. D Block Periodic Table Definition.
From cabinet.matttroy.net
Periodic Table Of Elements With Names And Symbols In Order D Block Periodic Table Definition These elements are all metals, usually with two or more oxidation states. Their general electron configuration is [noblegas] (n −1) d1−10ns0−2. Transition elements are those elements that have partially or incompletely filled d orbital in their ground state or the most stable oxidation state. They are called so because the highest energy subshell, i.e., the subshell where the last electron. D Block Periodic Table Definition.
From sciencenotes.org
S Block Elements and Their Properties D Block Periodic Table Definition The “d” stands for “diffuse” and the azimuthal quantum number is 2. What are d block elements in chemistry? This is why they are called “transition” metals. Their general electron configuration is [noblegas] (n −1) d1−10ns0−2. They are called so because the highest energy subshell, i.e., the subshell where the last electron occupies, is a d subshell These elements are. D Block Periodic Table Definition.
From sciencenotes.org
Periodic Table Blocks of Elements D Block Periodic Table Definition These elements are all metals, usually with two or more oxidation states. Their general electron configuration is [noblegas] (n −1) d1−10ns0−2. The “d” stands for “diffuse” and the azimuthal quantum number is 2. They are called so because the highest energy subshell, i.e., the subshell where the last electron occupies, is a d subshell Transition elements are those elements that. D Block Periodic Table Definition.
From www.ck12.org
Families and Periods of the Periodic Table CK12 Foundation D Block Periodic Table Definition Their general electron configuration is [noblegas] (n −1) d1−10ns0−2. The “d” stands for “diffuse” and the azimuthal quantum number is 2. They are called so because the highest energy subshell, i.e., the subshell where the last electron occupies, is a d subshell This is why they are called “transition” metals. Transition elements are those elements that have partially or incompletely. D Block Periodic Table Definition.
From exolwbzog.blob.core.windows.net
What Element Is B at Bobby Daniels blog D Block Periodic Table Definition Their general electron configuration is [noblegas] (n −1) d1−10ns0−2. Transition elements are those elements that have partially or incompletely filled d orbital in their ground state or the most stable oxidation state. They are called so because the highest energy subshell, i.e., the subshell where the last electron occupies, is a d subshell What are d block elements in chemistry?. D Block Periodic Table Definition.
From www.priyamstudycentre.com
f block Elements Lanthanides and Actinides Periodic Table D Block Periodic Table Definition This is why they are called “transition” metals. Their general electron configuration is [noblegas] (n −1) d1−10ns0−2. They are called so because the highest energy subshell, i.e., the subshell where the last electron occupies, is a d subshell Transition elements are those elements that have partially or incompletely filled d orbital in their ground state or the most stable oxidation. D Block Periodic Table Definition.
From animalia-life.club
Representative Elements Periodic Table D Block Periodic Table Definition Transition elements are those elements that have partially or incompletely filled d orbital in their ground state or the most stable oxidation state. They are called so because the highest energy subshell, i.e., the subshell where the last electron occupies, is a d subshell The “d” stands for “diffuse” and the azimuthal quantum number is 2. This is why they. D Block Periodic Table Definition.