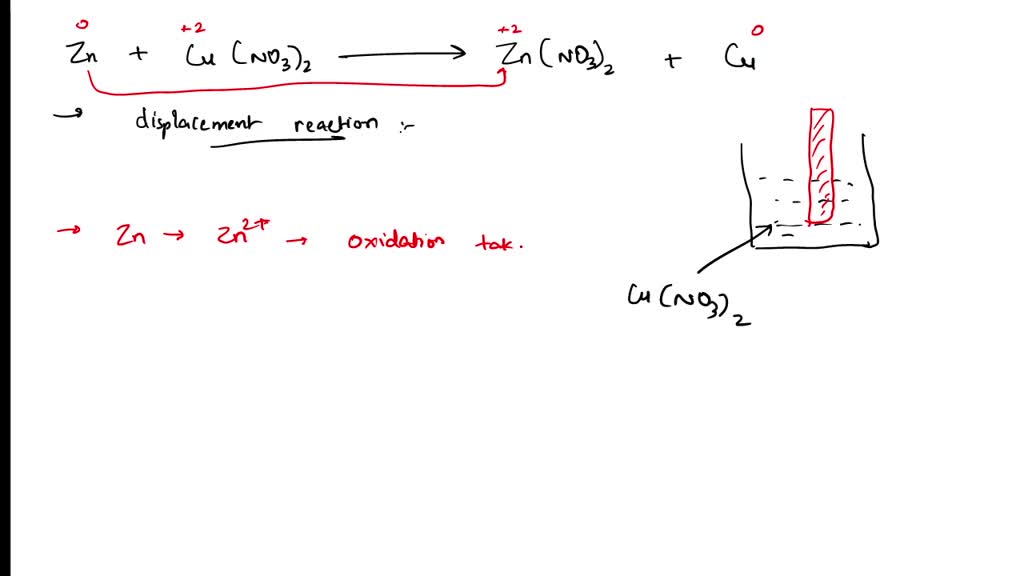
Zinc And Aqueous Copper (Ii) Sulfate . It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. Common polyatomic ion the topic of acids and polyatomic ions, there is nomenclature of aqueous acids. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). For each element, we check if the number of atoms is balanced on both sides. First, we set all coefficients to 1: If left in the solution for a longer. 1 zn + 1 cuso 4 = 1 znso 4 + 1 cu. Such acids include sulfuric acid. Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate.
from www.numerade.com
First, we set all coefficients to 1: Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). If left in the solution for a longer. It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Such acids include sulfuric acid. Common polyatomic ion the topic of acids and polyatomic ions, there is nomenclature of aqueous acids. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. 1 zn + 1 cuso 4 = 1 znso 4 + 1 cu.
SOLVED When zinc metal is placed into copper(II) nitrate solution
Zinc And Aqueous Copper (Ii) Sulfate It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. For each element, we check if the number of atoms is balanced on both sides. If left in the solution for a longer. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. 1 zn + 1 cuso 4 = 1 znso 4 + 1 cu. There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. First, we set all coefficients to 1: It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. Such acids include sulfuric acid. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. Common polyatomic ion the topic of acids and polyatomic ions, there is nomenclature of aqueous acids.
From www.numerade.com
SOLVED A reaction of solid zinc with aqueous copper II sulfate in a Zinc And Aqueous Copper (Ii) Sulfate For each element, we check if the number of atoms is balanced on both sides. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is. Zinc And Aqueous Copper (Ii) Sulfate.
From www.youtube.com
Mossy Zinc and Copper(II) Sulfate Solution YouTube Zinc And Aqueous Copper (Ii) Sulfate Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. Such acids include sulfuric acid. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper. Zinc And Aqueous Copper (Ii) Sulfate.
From www.youtube.com
The Reaction of Zinc Metal and Aqueous Copper(II) YouTube Zinc And Aqueous Copper (Ii) Sulfate First, we set all coefficients to 1: It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. 1 zn + 1 cuso 4 = 1 znso 4 + 1 cu. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution).. Zinc And Aqueous Copper (Ii) Sulfate.
From www.coursehero.com
[Solved] For the reaction between zinc and copper(II) sulfate, describe Zinc And Aqueous Copper (Ii) Sulfate It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. First, we set all coefficients to 1: When a strip of zinc. Zinc And Aqueous Copper (Ii) Sulfate.
From www.slideserve.com
PPT Ch. 8 Chemical Equations and Reactions PowerPoint Presentation Zinc And Aqueous Copper (Ii) Sulfate First, we set all coefficients to 1: When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. If left in the. Zinc And Aqueous Copper (Ii) Sulfate.
From www.sciencephoto.com
Zinc Reacting With Copper Sulfate Stock Image C028/0148 Science Zinc And Aqueous Copper (Ii) Sulfate Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). First, we set all coefficients to 1: There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. Such acids include sulfuric acid. It is commonly known that when zinc metal is placed in a solution of copper. Zinc And Aqueous Copper (Ii) Sulfate.
From shop.bioticsresearch.com
Aqueous Zinc™ Biotics Research Zinc And Aqueous Copper (Ii) Sulfate Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Common polyatomic ion the topic of acids and polyatomic ions, there is nomenclature of aqueous acids. If left in. Zinc And Aqueous Copper (Ii) Sulfate.
From www.chegg.com
Solved (6pts) Reaction A Zinc Metal, Zn(s), and Copper(II) Zinc And Aqueous Copper (Ii) Sulfate There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. Common polyatomic ion the topic of acids and polyatomic ions, there is nomenclature of aqueous acids. 1 zn + 1 cuso 4 = 1 znso 4 + 1 cu. When a strip of zinc metal is placed into a. Zinc And Aqueous Copper (Ii) Sulfate.
From www.researchgate.net
(PDF) Aqueous Zinc Sulfate Flow Through a Copper Mesh Anode Improves Zinc And Aqueous Copper (Ii) Sulfate It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. First, we set all coefficients to 1: Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. There are three main steps for writing the net ionic equation for zn +. Zinc And Aqueous Copper (Ii) Sulfate.
From oneclass.com
OneClass Zinc and Copper(II) Sulfate Materials Two test tube rack, 1 Zinc And Aqueous Copper (Ii) Sulfate There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). Zinc + cupric sulfate = dinuclear copper. Zinc And Aqueous Copper (Ii) Sulfate.
From www.youtube.com
Zinc and Copper II Chloride YouTube Zinc And Aqueous Copper (Ii) Sulfate Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. Common polyatomic ion the topic of acids and polyatomic ions, there is nomenclature of aqueous acids. It is commonly known that when zinc metal is placed in. Zinc And Aqueous Copper (Ii) Sulfate.
From users.highland.edu
Describing Electrochemical Cells Zinc And Aqueous Copper (Ii) Sulfate Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. 1 zn + 1 cuso 4 = 1 znso 4 + 1 cu. If left in the solution for a longer. First, we set all coefficients to 1: For each element, we check if the number of atoms is balanced. Zinc And Aqueous Copper (Ii) Sulfate.
From www.alamy.com
Copper(II) sulfate and Zinc Powder in test tube with plug cap. Cosmetic Zinc And Aqueous Copper (Ii) Sulfate When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). If left in the solution for a longer. First, we set all coefficients to 1: 1 zn + 1. Zinc And Aqueous Copper (Ii) Sulfate.
From chem2u.blogspot.com
chem2U Electrolysis of Copper(II) Sulphate Zinc And Aqueous Copper (Ii) Sulfate If left in the solution for a longer. Such acids include sulfuric acid. Common polyatomic ion the topic of acids and polyatomic ions, there is nomenclature of aqueous acids. 1 zn + 1 cuso 4 = 1 znso 4 + 1 cu. There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 +. Zinc And Aqueous Copper (Ii) Sulfate.
From www.sciencephoto.com
Zinc, Copper Sulphate Reaction, 2 of 2 Stock Image C030/7891 Zinc And Aqueous Copper (Ii) Sulfate Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. 1 zn + 1 cuso 4 = 1. Zinc And Aqueous Copper (Ii) Sulfate.
From www.flinnsci.com
Copper(II) Sulfate, Powder, Lab Grade, 500 g Flinn Scientific Zinc And Aqueous Copper (Ii) Sulfate Such acids include sulfuric acid. If left in the solution for a longer. It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a. Zinc And Aqueous Copper (Ii) Sulfate.
From www.sciencephoto.com
Hydrated copper (II) sulphate crystals Stock Image C004/7690 Zinc And Aqueous Copper (Ii) Sulfate Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). First, we set all coefficients to 1: Such acids include sulfuric acid. Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is. Zinc And Aqueous Copper (Ii) Sulfate.
From www.academia.edu
(PDF) Heat of reaction the zinc / copper(II) sulfate(VI) reaction Why Zinc And Aqueous Copper (Ii) Sulfate Common polyatomic ion the topic of acids and polyatomic ions, there is nomenclature of aqueous acids. First, we set all coefficients to 1: Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. If left in the solution for a longer. It is commonly known that when zinc metal is. Zinc And Aqueous Copper (Ii) Sulfate.
From www.youtube.com
Copper (II) sulfate and zinc reactions YouTube Zinc And Aqueous Copper (Ii) Sulfate There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Such acids include sulfuric acid. First, we set all coefficients. Zinc And Aqueous Copper (Ii) Sulfate.
From fphoto.photoshelter.com
science chemistry redox reaction zinc Fundamental Photographs The Zinc And Aqueous Copper (Ii) Sulfate For each element, we check if the number of atoms is balanced on both sides. 1 zn + 1 cuso 4 = 1 znso 4 + 1 cu. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. Such acids include sulfuric acid. Common polyatomic ion the topic of acids. Zinc And Aqueous Copper (Ii) Sulfate.
From www.numerade.com
SOLVED When zinc metal is placed into copper(II) nitrate solution Zinc And Aqueous Copper (Ii) Sulfate Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. First, we set all coefficients to 1: For each element, we check if the number of atoms is balanced on both sides. There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. Zinc (zn) is more reactive than. Zinc And Aqueous Copper (Ii) Sulfate.
From www.sciencephoto.com
Zinc Reacting With Copper Sulfate Stock Image C028/0146 Science Zinc And Aqueous Copper (Ii) Sulfate Such acids include sulfuric acid. Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. First, we set all coefficients to 1: There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate. Zinc And Aqueous Copper (Ii) Sulfate.
From www.reddit.com
Copper(II) sulfate r/MineralPorn Zinc And Aqueous Copper (Ii) Sulfate Common polyatomic ion the topic of acids and polyatomic ions, there is nomenclature of aqueous acids. It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). For each element, we. Zinc And Aqueous Copper (Ii) Sulfate.
From flatworldknowledge.lardbucket.org
Describing Electrochemical Cells Zinc And Aqueous Copper (Ii) Sulfate Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. If left in the solution for a longer. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution.. Zinc And Aqueous Copper (Ii) Sulfate.
From schoolworkhelper.net
Single Displacement Reactions Lab Explained SchoolWorkHelper Zinc And Aqueous Copper (Ii) Sulfate 1 zn + 1 cuso 4 = 1 znso 4 + 1 cu. For each element, we check if the number of atoms is balanced on both sides. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. If. Zinc And Aqueous Copper (Ii) Sulfate.
From www.orientjchem.org
Thermodynamic study of copper sulphate and zinc sulphate in water and Zinc And Aqueous Copper (Ii) Sulfate Such acids include sulfuric acid. If left in the solution for a longer. 1 zn + 1 cuso 4 = 1 znso 4 + 1 cu. There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu. Zinc And Aqueous Copper (Ii) Sulfate.
From www.youtube.com
Zinc + Copper Sulfate Reaction YouTube Zinc And Aqueous Copper (Ii) Sulfate Common polyatomic ion the topic of acids and polyatomic ions, there is nomenclature of aqueous acids. There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as. Zinc And Aqueous Copper (Ii) Sulfate.
From www.youtube.com
[4K] Displacement Reaction of Metals Zinc in Copper (II) Sulfate Zinc And Aqueous Copper (Ii) Sulfate Such acids include sulfuric acid. Common polyatomic ion the topic of acids and polyatomic ions, there is nomenclature of aqueous acids. Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to. Zinc And Aqueous Copper (Ii) Sulfate.
From www.numerade.com
SOLVED Write the balanced chemical equation, including the states, for Zinc And Aqueous Copper (Ii) Sulfate First, we set all coefficients to 1: 1 zn + 1 cuso 4 = 1 znso 4 + 1 cu. For each element, we check if the number of atoms is balanced on both sides. There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. Zn + cuso4 =. Zinc And Aqueous Copper (Ii) Sulfate.
From www.orientjchem.org
Thermodynamic study of copper sulphate and zinc sulphate in water and Zinc And Aqueous Copper (Ii) Sulfate Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. Common polyatomic ion the topic of acids and polyatomic ions, there is nomenclature of aqueous acids. Such acids include sulfuric acid. Zn + cuso4 = cu2 + znso4 is. Zinc And Aqueous Copper (Ii) Sulfate.
From www.numerade.com
SOLVED Write a balanced equation for the singlereplacement oxidation Zinc And Aqueous Copper (Ii) Sulfate If left in the solution for a longer. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. For each element, we check if the number of atoms is balanced on both sides. There are three main steps for. Zinc And Aqueous Copper (Ii) Sulfate.
From www.chegg.com
Solved Suppose you are reacting zinc metal with aqueous Zinc And Aqueous Copper (Ii) Sulfate There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. For each element, we check if the number of atoms is balanced on both sides. It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper. Zinc And Aqueous Copper (Ii) Sulfate.
From cristianmeowterry.blogspot.com
Molecular Equation for Copper Ii Sulfate and Sodium Phosphate Zinc And Aqueous Copper (Ii) Sulfate Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. 1 zn + 1 cuso 4 = 1 znso 4 + 1 cu. Such acids include sulfuric acid. It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. For each element,. Zinc And Aqueous Copper (Ii) Sulfate.
From www.youtube.com
Net Ionic Equation for Zn + CuSO4 Zinc + Copper (II) Sulfate YouTube Zinc And Aqueous Copper (Ii) Sulfate For each element, we check if the number of atoms is balanced on both sides. Common polyatomic ion the topic of acids and polyatomic ions, there is nomenclature of aqueous acids. If left in the solution for a longer. There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +.. Zinc And Aqueous Copper (Ii) Sulfate.
From uwaterloo.ca
Zinc metal in a solution of copper(II) sulfate and sulfuric acid from Zinc And Aqueous Copper (Ii) Sulfate Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. 1 zn + 1 cuso 4 = 1 znso 4 + 1 cu. There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). Zinc (zn) is. Zinc And Aqueous Copper (Ii) Sulfate.