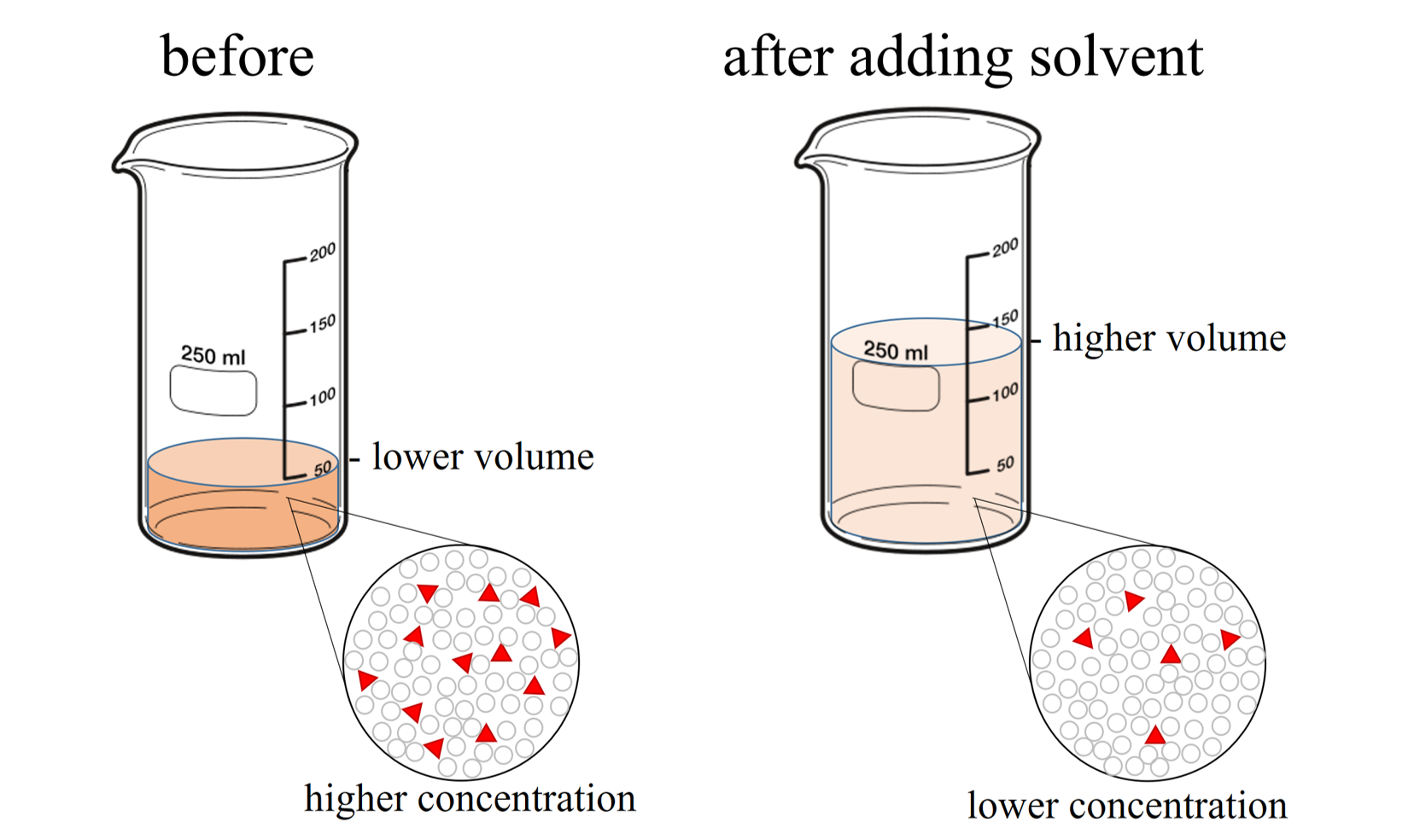
Dilute Of Solution . Of course, the resulting solution is. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. Often, a worker will need to change the concentration of a. the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. — learning objective. to dilute a solution means to add more solvent without the addition of more solute. dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Learn how to dilute and concentrate solutions. — the solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain an arbitrary.
from chem.libretexts.org
to dilute a solution means to add more solvent without the addition of more solute. Learn how to dilute and concentrate solutions. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. Of course, the resulting solution is. — the solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain an arbitrary. — learning objective. the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. Often, a worker will need to change the concentration of a. Dilution is a process used to lower the concentration of the original solution by adding more solvent. dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the.
14.7 Solution Dilution Chemistry LibreTexts
Dilute Of Solution Often, a worker will need to change the concentration of a. Learn how to dilute and concentrate solutions. the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. — the solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain an arbitrary. dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. — learning objective. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Often, a worker will need to change the concentration of a. Of course, the resulting solution is. to dilute a solution means to add more solvent without the addition of more solute. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more.
From www.medicine.mcgill.ca
Serial Dilutions Dilute Of Solution to dilute a solution means to add more solvent without the addition of more solute. Learn how to dilute and concentrate solutions. the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. — learning objective. — the solution dilution calculator will calculate for you how to dilute a stock. Dilute Of Solution.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Dilute Of Solution Dilution is a process used to lower the concentration of the original solution by adding more solvent. to dilute a solution means to add more solvent without the addition of more solute. Of course, the resulting solution is. Often, a worker will need to change the concentration of a. dilution is the process of decreasing the concentration of. Dilute Of Solution.
From brainly.ph
What is the difference between a dilute solution and a concentrated Dilute Of Solution — learning objective. to dilute a solution means to add more solvent without the addition of more solute. — the solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain an arbitrary. Dilution is a process used to lower the concentration of the original solution by adding more solvent.. Dilute Of Solution.
From www.youtube.com
Dilute and Concentrated Solution YouTube Dilute Of Solution to dilute a solution means to add more solvent without the addition of more solute. Learn how to dilute and concentrate solutions. the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. Of course, the resulting solution is. Dilution is a process used to lower the concentration of the original solution. Dilute Of Solution.
From www.geeksforgeeks.org
Concentration of Solution Definition, Formulas & Solved Examples Dilute Of Solution — the solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain an arbitrary. Dilution is a process used to lower the concentration of the original solution by adding more solvent. — learning objective. dilution is the process of decreasing the concentration of a solute in a solution, usually. Dilute Of Solution.
From ar.inspiredpencil.com
Dilute Solution Diagram Dilute Of Solution — the solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain an arbitrary. the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. — learning objective. to dilute a solution means to add more solvent without the addition of more. Dilute Of Solution.
From www.youtube.com
Dilute, Concentrated and saturated solution YouTube Dilute Of Solution Dilution is a process used to lower the concentration of the original solution by adding more solvent. Of course, the resulting solution is. dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. to dilute a solution means to add more solvent without the addition of more. Dilute Of Solution.
From quizlet.com
Mixtures and Solutions Diagram Quizlet Dilute Of Solution to dilute a solution means to add more solvent without the addition of more solute. dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. Often,. Dilute Of Solution.
From exoixldni.blob.core.windows.net
Diluted Standard Method For Related Substances at Lydia Hudson blog Dilute Of Solution dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. Learn how to dilute and concentrate solutions. to dilute a solution means to add more solvent without the addition of more solute. Dilution is a process used to lower the concentration of the original solution by adding more. Dilute Of Solution.
From www.showme.com
Concentrated vs. Diluted Solution Science ShowMe Dilute Of Solution Learn how to dilute and concentrate solutions. to dilute a solution means to add more solvent without the addition of more solute. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Often, a worker will need to change the concentration of a. dilution is the process of decreasing the concentration. Dilute Of Solution.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilute Of Solution — the solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain an arbitrary. Learn how to dilute and concentrate solutions. the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. Dilution is a process used to lower the concentration of the original. Dilute Of Solution.
From www.askiitians.com
Types Of Solutions Study Material for IIT JEE askIITians Dilute Of Solution Learn how to dilute and concentrate solutions. to dilute a solution means to add more solvent without the addition of more solute. — learning objective. the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. Dilution is a process used to lower the concentration of the original solution by adding. Dilute Of Solution.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and Dilute Of Solution Often, a worker will need to change the concentration of a. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. the solution on the right. Dilute Of Solution.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilute Of Solution — learning objective. dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. to dilute a solution means to add more solvent without the addition of more solute. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by. Dilute Of Solution.
From stock.adobe.com
A concentrated solution is one that has a relatively large amount of Dilute Of Solution dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. to dilute a solution means to add more solvent without the addition of more solute. the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. Learn how to dilute and. Dilute Of Solution.
From www.youtube.com
Dilution, solution, ratio, proportion (laboratory. calculations) YouTube Dilute Of Solution — learning objective. to dilute a solution means to add more solvent without the addition of more solute. Often, a worker will need to change the concentration of a. the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. Of course, the resulting solution is. dilution is the process. Dilute Of Solution.
From exosuagel.blob.core.windows.net
Solution Dilution Calculator Physiology at Mike Wang blog Dilute Of Solution — learning objective. Of course, the resulting solution is. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Often, a worker will need to change the concentration of a. Learn how to dilute and concentrate solutions. dilution is the process of decreasing the concentration of a solute in a solution,. Dilute Of Solution.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Dilute Of Solution Often, a worker will need to change the concentration of a. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Learn how to dilute and concentrate solutions. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. — learning objective.. Dilute Of Solution.
From www.pinterest.com
Dilute Flashcards, Easy science, Science student Dilute Of Solution Dilution is a process used to lower the concentration of the original solution by adding more solvent. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. Often, a worker will need to change the concentration of a. — the solution dilution calculator will calculate for you how. Dilute Of Solution.
From www.pinterest.cl
Dilution when solvent is added to dilute a solution, the number of Dilute Of Solution dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a. Dilution is a process used to lower the concentration of the original solution by adding more solvent. dilution is. Dilute Of Solution.
From dxoaudgjb.blob.core.windows.net
Dilute Solution Meaning Simple at James Flynn blog Dilute Of Solution to dilute a solution means to add more solvent without the addition of more solute. Often, a worker will need to change the concentration of a. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. — the solution dilution calculator will calculate for you how to. Dilute Of Solution.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilute Of Solution Of course, the resulting solution is. to dilute a solution means to add more solvent without the addition of more solute. Learn how to dilute and concentrate solutions. Dilution is a process used to lower the concentration of the original solution by adding more solvent. — the solution dilution calculator will calculate for you how to dilute a. Dilute Of Solution.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube Dilute Of Solution Learn how to dilute and concentrate solutions. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Often, a worker will need to change the concentration of a. — the solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain an arbitrary. to. Dilute Of Solution.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Dilute Of Solution Learn how to dilute and concentrate solutions. the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. Often, a worker will need to change the concentration of a. — learning objective. to dilute a solution means to add more solvent without the addition of more solute. Of course, the resulting. Dilute Of Solution.
From twinklsecondary.blog
Products of a Dilution Series A Level Biology Revision Dilute Of Solution Learn how to dilute and concentrate solutions. — the solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain an arbitrary. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. Dilution is a process used to lower the concentration. Dilute Of Solution.
From www.youtube.com
U10L4 Molarity, Dilution, PPM, and Molality Calculations YouTube Dilute Of Solution Dilution is a process used to lower the concentration of the original solution by adding more solvent. — the solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain an arbitrary. dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent. Dilute Of Solution.
From exozlhxkx.blob.core.windows.net
Water Dilute Solution at Faustina Baptist blog Dilute Of Solution the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. — learning objective. dilution is the process of decreasing the concentration of a solute in a solution, usually. Dilute Of Solution.
From slidetodoc.com
WATER AND SOLUTIONS A solution is a mixture Dilute Of Solution — learning objective. to dilute a solution means to add more solvent without the addition of more solute. Dilution is a process used to lower the concentration of the original solution by adding more solvent. the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. Learn how to dilute and. Dilute Of Solution.
From general.chemistrysteps.com
Dilution of a Stock Solution and Calculations Based Morality Dilute Of Solution the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. — learning objective. Dilution is a process used to lower the concentration of the original solution by adding more solvent. — the solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain. Dilute Of Solution.
From www.slideserve.com
PPT Reaction Stoichiometry Mole Method Calculations PowerPoint Dilute Of Solution Dilution is a process used to lower the concentration of the original solution by adding more solvent. Learn how to dilute and concentrate solutions. the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. dilution is the process of “lowering the concentration of a solute in a solution by simply adding. Dilute Of Solution.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilute Of Solution the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. — the solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain an arbitrary. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Of course,. Dilute Of Solution.
From www.youtube.com
What is Dilute Solution? Chemistry YouTube Dilute Of Solution — learning objective. Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a. dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. — the solution dilution calculator will calculate for you how to dilute a stock solution. Dilute Of Solution.
From fphoto.photoshelter.com
science chemistry solubility dilution Fundamental Photographs The Dilute Of Solution Of course, the resulting solution is. Learn how to dilute and concentrate solutions. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. Often, a worker will need to change the concentration of a. the solution on the right is more dilute because the copper nitrate is dissolved. Dilute Of Solution.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions Dilute Of Solution — the solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain an arbitrary. to dilute a solution means to add more solvent without the addition of more solute. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Of course, the resulting. Dilute Of Solution.
From www.slideserve.com
PPT Diluting Solutions PowerPoint Presentation, free download ID Dilute Of Solution Learn how to dilute and concentrate solutions. — the solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain an arbitrary. Often, a worker will need to change the concentration of a. Dilution is a process used to lower the concentration of the original solution by adding more solvent. to. Dilute Of Solution.