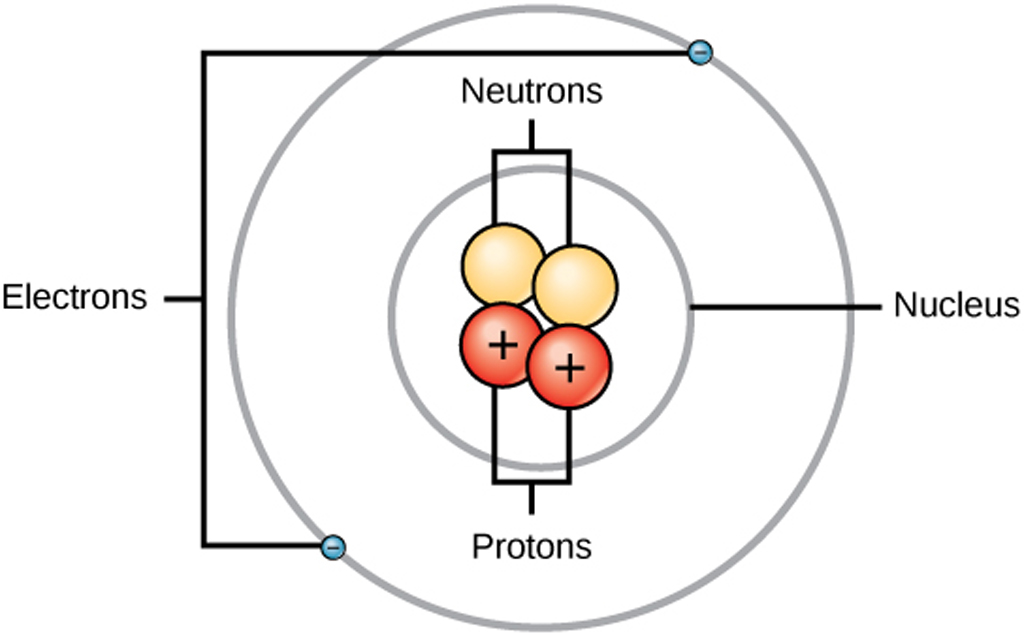
What Gives An Atom Its Volume . the calculation of the volume of an atom is based on a simplistic understanding of an atom as a sphere. if an atom has more or fewer electrons than its atomic number, then it becomes respectively negatively or positively. in volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. rutherford's gold foil experiment has proved that most of the mass of an atom is concentrated in the nucleus alone. atomic volume refers to the spatial dimension of an atom, which is determined by the van der waals radius or the. almost all of the volume of an atom consists of empty space in which electrons, the fundamental carriers of negative electric. the volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus.
from www.electronicsandyou.com
in volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. rutherford's gold foil experiment has proved that most of the mass of an atom is concentrated in the nucleus alone. the calculation of the volume of an atom is based on a simplistic understanding of an atom as a sphere. if an atom has more or fewer electrons than its atomic number, then it becomes respectively negatively or positively. the volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus. almost all of the volume of an atom consists of empty space in which electrons, the fundamental carriers of negative electric. atomic volume refers to the spatial dimension of an atom, which is determined by the van der waals radius or the.
Structure of an Atom Structure & Use of Electron & Proton in Electronics
What Gives An Atom Its Volume the calculation of the volume of an atom is based on a simplistic understanding of an atom as a sphere. the volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus. rutherford's gold foil experiment has proved that most of the mass of an atom is concentrated in the nucleus alone. in volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. almost all of the volume of an atom consists of empty space in which electrons, the fundamental carriers of negative electric. if an atom has more or fewer electrons than its atomic number, then it becomes respectively negatively or positively. atomic volume refers to the spatial dimension of an atom, which is determined by the van der waals radius or the. the calculation of the volume of an atom is based on a simplistic understanding of an atom as a sphere.
From www.sciencefacts.net
Atom Definition, Structure & Parts with Labeled Diagram What Gives An Atom Its Volume if an atom has more or fewer electrons than its atomic number, then it becomes respectively negatively or positively. in volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. the volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus.. What Gives An Atom Its Volume.
From www.sciencefacts.net
Atom Definition, Structure & Parts with Labeled Diagram What Gives An Atom Its Volume almost all of the volume of an atom consists of empty space in which electrons, the fundamental carriers of negative electric. if an atom has more or fewer electrons than its atomic number, then it becomes respectively negatively or positively. atomic volume refers to the spatial dimension of an atom, which is determined by the van der. What Gives An Atom Its Volume.
From www.pathwaystochemistry.com
Atomic Structure Pathways to Chemistry What Gives An Atom Its Volume almost all of the volume of an atom consists of empty space in which electrons, the fundamental carriers of negative electric. atomic volume refers to the spatial dimension of an atom, which is determined by the van der waals radius or the. if an atom has more or fewer electrons than its atomic number, then it becomes. What Gives An Atom Its Volume.
From www.youtube.com
Atomic volume is YouTube What Gives An Atom Its Volume rutherford's gold foil experiment has proved that most of the mass of an atom is concentrated in the nucleus alone. almost all of the volume of an atom consists of empty space in which electrons, the fundamental carriers of negative electric. if an atom has more or fewer electrons than its atomic number, then it becomes respectively. What Gives An Atom Its Volume.
From brainly.in
the volume of an atom is provided in some way by its...... Brainly.in What Gives An Atom Its Volume in volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. atomic volume refers to the spatial dimension of an atom, which is determined by the van der waals radius or the. the calculation of the volume of an atom is based on a simplistic understanding of an. What Gives An Atom Its Volume.
From www.mindomo.com
Energy Mind Map What Gives An Atom Its Volume almost all of the volume of an atom consists of empty space in which electrons, the fundamental carriers of negative electric. the volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus. if an atom has more or fewer electrons than its atomic number, then it becomes respectively negatively or. What Gives An Atom Its Volume.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer What Gives An Atom Its Volume atomic volume refers to the spatial dimension of an atom, which is determined by the van der waals radius or the. almost all of the volume of an atom consists of empty space in which electrons, the fundamental carriers of negative electric. the calculation of the volume of an atom is based on a simplistic understanding of. What Gives An Atom Its Volume.
From vhmsscience.weebly.com
Atomic Structures & the Periodic Table VISTA HEIGHTS 8TH GRADE SCIENCE What Gives An Atom Its Volume rutherford's gold foil experiment has proved that most of the mass of an atom is concentrated in the nucleus alone. almost all of the volume of an atom consists of empty space in which electrons, the fundamental carriers of negative electric. the volume of an atom is about 15 orders of magnitude larger than the volume of. What Gives An Atom Its Volume.
From www.biologyonline.com
Atom Definition and Examples Biology Online Dictionary What Gives An Atom Its Volume almost all of the volume of an atom consists of empty space in which electrons, the fundamental carriers of negative electric. the volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus. in volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part. What Gives An Atom Its Volume.
From sciencenotes.org
Learn the Parts of an Atom What Gives An Atom Its Volume if an atom has more or fewer electrons than its atomic number, then it becomes respectively negatively or positively. the volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus. the calculation of the volume of an atom is based on a simplistic understanding of an atom as a sphere.. What Gives An Atom Its Volume.
From www.thoughtco.com
Basic Model of the Atom Atomic Theory What Gives An Atom Its Volume the calculation of the volume of an atom is based on a simplistic understanding of an atom as a sphere. if an atom has more or fewer electrons than its atomic number, then it becomes respectively negatively or positively. in volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part. What Gives An Atom Its Volume.
From www.thesciencehive.co.uk
Atomic Structure* — the science sauce What Gives An Atom Its Volume atomic volume refers to the spatial dimension of an atom, which is determined by the van der waals radius or the. the calculation of the volume of an atom is based on a simplistic understanding of an atom as a sphere. the volume of an atom is about 15 orders of magnitude larger than the volume of. What Gives An Atom Its Volume.
From chem.libretexts.org
Chapter 1.5 The Atom Chemistry LibreTexts What Gives An Atom Its Volume atomic volume refers to the spatial dimension of an atom, which is determined by the van der waals radius or the. in volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. rutherford's gold foil experiment has proved that most of the mass of an atom is concentrated. What Gives An Atom Its Volume.
From www.britannica.com
Basic concept and structure of an atom Britannica What Gives An Atom Its Volume the volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus. rutherford's gold foil experiment has proved that most of the mass of an atom is concentrated in the nucleus alone. almost all of the volume of an atom consists of empty space in which electrons, the fundamental carriers of. What Gives An Atom Its Volume.
From www.slideserve.com
PPT Section 4.2—Atomic Structure PowerPoint Presentation, free What Gives An Atom Its Volume almost all of the volume of an atom consists of empty space in which electrons, the fundamental carriers of negative electric. if an atom has more or fewer electrons than its atomic number, then it becomes respectively negatively or positively. the calculation of the volume of an atom is based on a simplistic understanding of an atom. What Gives An Atom Its Volume.
From www.youtube.com
Finding radius and volume of atom from density and molar mass YouTube What Gives An Atom Its Volume the calculation of the volume of an atom is based on a simplistic understanding of an atom as a sphere. almost all of the volume of an atom consists of empty space in which electrons, the fundamental carriers of negative electric. if an atom has more or fewer electrons than its atomic number, then it becomes respectively. What Gives An Atom Its Volume.
From www.facebook.com
What Gives an Atom its Volume? In the universe, every mass defect is What Gives An Atom Its Volume in volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. rutherford's gold foil experiment has proved that most of the mass of an atom is concentrated in the nucleus alone. the volume of an atom is about 15 orders of magnitude larger than the volume of a. What Gives An Atom Its Volume.
From www.slideserve.com
PPT The Nucleus of the Atom PowerPoint Presentation, free download What Gives An Atom Its Volume atomic volume refers to the spatial dimension of an atom, which is determined by the van der waals radius or the. the volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus. if an atom has more or fewer electrons than its atomic number, then it becomes respectively negatively or. What Gives An Atom Its Volume.
From mungfali.com
Periodic Table With Nucleon Number What Gives An Atom Its Volume in volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. almost all of the volume of an atom consists of empty space in which electrons, the fundamental carriers of negative electric. atomic volume refers to the spatial dimension of an atom, which is determined by the van. What Gives An Atom Its Volume.
From www.thoughtco.com
The Definition of Atomic Volume and How to Calculate It What Gives An Atom Its Volume the volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus. in volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. atomic volume refers to the spatial dimension of an atom, which is determined by the van der waals radius. What Gives An Atom Its Volume.
From www.geeksforgeeks.org
Atomic Mass Table of First 30 Elements What Gives An Atom Its Volume the volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus. if an atom has more or fewer electrons than its atomic number, then it becomes respectively negatively or positively. rutherford's gold foil experiment has proved that most of the mass of an atom is concentrated in the nucleus alone.. What Gives An Atom Its Volume.
From www.broadlearnings.com
Atomic Structure Broad Learnings What Gives An Atom Its Volume atomic volume refers to the spatial dimension of an atom, which is determined by the van der waals radius or the. in volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. the calculation of the volume of an atom is based on a simplistic understanding of an. What Gives An Atom Its Volume.
From oerpub.github.io
The top panel of this figure shows two electrons orbiting around the What Gives An Atom Its Volume in volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. the volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus. if an atom has more or fewer electrons than its atomic number, then it becomes respectively negatively or positively.. What Gives An Atom Its Volume.
From www.worksheetsplanet.com
What is an Atom Meaning & Definition of Atom What Gives An Atom Its Volume in volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. if an atom has more or fewer electrons than its atomic number, then it becomes respectively negatively or positively. almost all of the volume of an atom consists of empty space in which electrons, the fundamental carriers. What Gives An Atom Its Volume.
From spmscience.blog.onlinetuition.com.my
4.2 Structure of Atoms SPM Science What Gives An Atom Its Volume if an atom has more or fewer electrons than its atomic number, then it becomes respectively negatively or positively. atomic volume refers to the spatial dimension of an atom, which is determined by the van der waals radius or the. rutherford's gold foil experiment has proved that most of the mass of an atom is concentrated in. What Gives An Atom Its Volume.
From mavink.com
Components Of An Atom Diagram What Gives An Atom Its Volume the calculation of the volume of an atom is based on a simplistic understanding of an atom as a sphere. the volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus. atomic volume refers to the spatial dimension of an atom, which is determined by the van der waals radius. What Gives An Atom Its Volume.
From sciencing.com
How to Calculate the Volume of an Atom Sciencing What Gives An Atom Its Volume almost all of the volume of an atom consists of empty space in which electrons, the fundamental carriers of negative electric. in volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. if an atom has more or fewer electrons than its atomic number, then it becomes respectively. What Gives An Atom Its Volume.
From kidspressmagazine.com
The Structure of Atoms What Gives An Atom Its Volume almost all of the volume of an atom consists of empty space in which electrons, the fundamental carriers of negative electric. the volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus. if an atom has more or fewer electrons than its atomic number, then it becomes respectively negatively or. What Gives An Atom Its Volume.
From mmerevise.co.uk
Atoms Questions and Revision MME What Gives An Atom Its Volume the calculation of the volume of an atom is based on a simplistic understanding of an atom as a sphere. the volume of an atom is about 15 orders of magnitude larger than the volume of a nucleus. in volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in. What Gives An Atom Its Volume.
From www.slideserve.com
PPT Subatomic Particles PowerPoint Presentation, free download ID What Gives An Atom Its Volume the calculation of the volume of an atom is based on a simplistic understanding of an atom as a sphere. rutherford's gold foil experiment has proved that most of the mass of an atom is concentrated in the nucleus alone. if an atom has more or fewer electrons than its atomic number, then it becomes respectively negatively. What Gives An Atom Its Volume.
From circuitlibrarycorti.z13.web.core.windows.net
Atomic Structure Diagram Labelled What Gives An Atom Its Volume in volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. atomic volume refers to the spatial dimension of an atom, which is determined by the van der waals radius or the. the calculation of the volume of an atom is based on a simplistic understanding of an. What Gives An Atom Its Volume.
From www.teachoo.com
What is Atom? How does it Exist? and it's Symbols Teachoo What Gives An Atom Its Volume rutherford's gold foil experiment has proved that most of the mass of an atom is concentrated in the nucleus alone. atomic volume refers to the spatial dimension of an atom, which is determined by the van der waals radius or the. the volume of an atom is about 15 orders of magnitude larger than the volume of. What Gives An Atom Its Volume.
From www.electronicsandyou.com
Structure of an Atom Structure & Use of Electron & Proton in Electronics What Gives An Atom Its Volume atomic volume refers to the spatial dimension of an atom, which is determined by the van der waals radius or the. the calculation of the volume of an atom is based on a simplistic understanding of an atom as a sphere. rutherford's gold foil experiment has proved that most of the mass of an atom is concentrated. What Gives An Atom Its Volume.
From www.youtube.com
CHM122 2_6_1 Volume of an Atom YouTube What Gives An Atom Its Volume rutherford's gold foil experiment has proved that most of the mass of an atom is concentrated in the nucleus alone. atomic volume refers to the spatial dimension of an atom, which is determined by the van der waals radius or the. if an atom has more or fewer electrons than its atomic number, then it becomes respectively. What Gives An Atom Its Volume.
From www.bartleby.com
Answered Most of the volume of an atom is made… bartleby What Gives An Atom Its Volume in volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. rutherford's gold foil experiment has proved that most of the mass of an atom is concentrated in the nucleus alone. the volume of an atom is about 15 orders of magnitude larger than the volume of a. What Gives An Atom Its Volume.