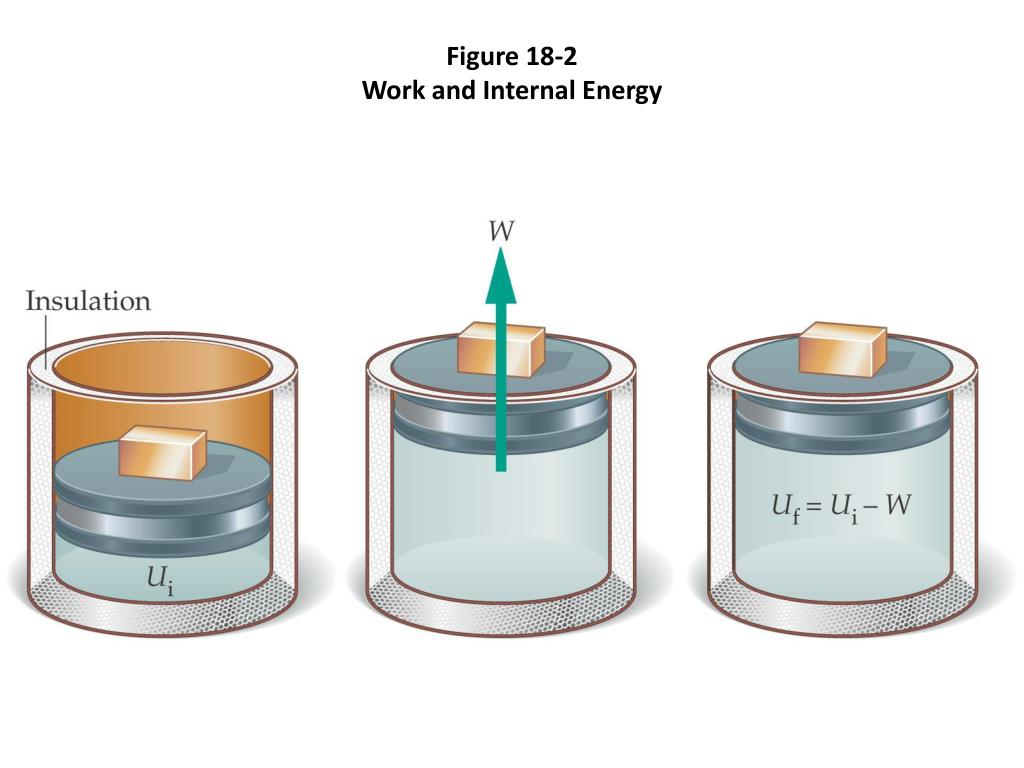
Ideal Gas Internal Energy . It can be due to the motion of its. Internal energy of an ideal gas. The internal energy of a gas is defined as: The internal energy of a gas is defined to be the total energy of the gas when the center of mass of the gas is at rest. At the same time, 140 j of energy is. If work is done on the gas energy is added to the gas. The internal energy of an ideal gas consists of only kinetic energy. In thermodynamics, the fact that the energy of an ideal gas depends only on temperature is an experimental observation from the free. The sum of the kinetic and potential energies of the particles inside the gas. Internal energy of an ideal gas. The internal energy u of a thermodynamic system is the energy it contains. A sample of an ideal gas in the cylinder of an engine is compressed from 400 ml to 50.0 ml during the compression stroke against a constant pressure of 8.00 atm. The internal energy consists of the.
from www.slideserve.com
A sample of an ideal gas in the cylinder of an engine is compressed from 400 ml to 50.0 ml during the compression stroke against a constant pressure of 8.00 atm. The internal energy of an ideal gas consists of only kinetic energy. Internal energy of an ideal gas. The internal energy u of a thermodynamic system is the energy it contains. The internal energy of a gas is defined to be the total energy of the gas when the center of mass of the gas is at rest. Internal energy of an ideal gas. It can be due to the motion of its. If work is done on the gas energy is added to the gas. The internal energy of a gas is defined as: The sum of the kinetic and potential energies of the particles inside the gas.
PPT Ideal gas PowerPoint Presentation, free download ID2134712
Ideal Gas Internal Energy A sample of an ideal gas in the cylinder of an engine is compressed from 400 ml to 50.0 ml during the compression stroke against a constant pressure of 8.00 atm. The internal energy consists of the. The internal energy of a gas is defined to be the total energy of the gas when the center of mass of the gas is at rest. If work is done on the gas energy is added to the gas. The sum of the kinetic and potential energies of the particles inside the gas. A sample of an ideal gas in the cylinder of an engine is compressed from 400 ml to 50.0 ml during the compression stroke against a constant pressure of 8.00 atm. The internal energy u of a thermodynamic system is the energy it contains. The internal energy of a gas is defined as: At the same time, 140 j of energy is. In thermodynamics, the fact that the energy of an ideal gas depends only on temperature is an experimental observation from the free. The internal energy of an ideal gas consists of only kinetic energy. Internal energy of an ideal gas. Internal energy of an ideal gas. It can be due to the motion of its.
From www.youtube.com
Thermodynamics and Fluid Mechanics C2 L13 Internal Energy of Ideal Gas Internal Energy The internal energy consists of the. Internal energy of an ideal gas. It can be due to the motion of its. The internal energy of an ideal gas consists of only kinetic energy. The sum of the kinetic and potential energies of the particles inside the gas. The internal energy u of a thermodynamic system is the energy it contains.. Ideal Gas Internal Energy.
From www.youtube.com
8.18 What is the internal energy of an ideal gas? YouTube Ideal Gas Internal Energy A sample of an ideal gas in the cylinder of an engine is compressed from 400 ml to 50.0 ml during the compression stroke against a constant pressure of 8.00 atm. If work is done on the gas energy is added to the gas. The sum of the kinetic and potential energies of the particles inside the gas. The internal. Ideal Gas Internal Energy.
From www.youtube.com
Lecture 13 Ideal Gases Internal Energy, Enthalpy, and Specific Heats Ideal Gas Internal Energy The internal energy of a gas is defined as: At the same time, 140 j of energy is. The internal energy u of a thermodynamic system is the energy it contains. The sum of the kinetic and potential energies of the particles inside the gas. The internal energy of a gas is defined to be the total energy of the. Ideal Gas Internal Energy.
From www.tec-science.com
Berechnung der inneren Energie für ideale Gase tecscience Ideal Gas Internal Energy The internal energy of a gas is defined to be the total energy of the gas when the center of mass of the gas is at rest. The internal energy u of a thermodynamic system is the energy it contains. The internal energy consists of the. If work is done on the gas energy is added to the gas. The. Ideal Gas Internal Energy.
From www.tec-science.com
Calculation of the internal energy for ideal gases tecscience Ideal Gas Internal Energy At the same time, 140 j of energy is. Internal energy of an ideal gas. The internal energy of an ideal gas consists of only kinetic energy. The sum of the kinetic and potential energies of the particles inside the gas. The internal energy consists of the. The internal energy of a gas is defined to be the total energy. Ideal Gas Internal Energy.
From www.slideserve.com
PPT The Ideal Gas PowerPoint Presentation, free download ID6789672 Ideal Gas Internal Energy If work is done on the gas energy is added to the gas. The internal energy of a gas is defined as: The internal energy u of a thermodynamic system is the energy it contains. It can be due to the motion of its. The internal energy of a gas is defined to be the total energy of the gas. Ideal Gas Internal Energy.
From www.youtube.com
Ideal Gases Specific Heat, Internal Energy, Enthalpy Thermodynamics Ideal Gas Internal Energy The internal energy of an ideal gas consists of only kinetic energy. The sum of the kinetic and potential energies of the particles inside the gas. The internal energy u of a thermodynamic system is the energy it contains. If work is done on the gas energy is added to the gas. The internal energy of a gas is defined. Ideal Gas Internal Energy.
From www.youtube.com
HTPIB15G Total Internal Energy of an Ideal Gas YouTube Ideal Gas Internal Energy At the same time, 140 j of energy is. Internal energy of an ideal gas. It can be due to the motion of its. The internal energy of a gas is defined to be the total energy of the gas when the center of mass of the gas is at rest. The internal energy u of a thermodynamic system is. Ideal Gas Internal Energy.
From www.tec-science.com
Calculation of the internal energy for ideal gases tecscience Ideal Gas Internal Energy At the same time, 140 j of energy is. It can be due to the motion of its. Internal energy of an ideal gas. The sum of the kinetic and potential energies of the particles inside the gas. The internal energy of a gas is defined to be the total energy of the gas when the center of mass of. Ideal Gas Internal Energy.
From www.researchgate.net
Internal energy distribution for the ideal gas rescaled by the size K Ideal Gas Internal Energy Internal energy of an ideal gas. The internal energy of a gas is defined to be the total energy of the gas when the center of mass of the gas is at rest. If work is done on the gas energy is added to the gas. The internal energy of an ideal gas consists of only kinetic energy. It can. Ideal Gas Internal Energy.
From www.toppr.com
The change in internal energy of an ideal gas when volume changes from Ideal Gas Internal Energy The sum of the kinetic and potential energies of the particles inside the gas. The internal energy consists of the. It can be due to the motion of its. The internal energy u of a thermodynamic system is the energy it contains. The internal energy of a gas is defined to be the total energy of the gas when the. Ideal Gas Internal Energy.
From www.youtube.com
Internal Energy of an Ideal Gas Molar Heat Capacity of Monatomic Ideal Gas Internal Energy The internal energy u of a thermodynamic system is the energy it contains. Internal energy of an ideal gas. The sum of the kinetic and potential energies of the particles inside the gas. The internal energy consists of the. Internal energy of an ideal gas. At the same time, 140 j of energy is. It can be due to the. Ideal Gas Internal Energy.
From www.youtube.com
Ideal gas mixture change in internal energy YouTube Ideal Gas Internal Energy The internal energy consists of the. The internal energy of a gas is defined as: The sum of the kinetic and potential energies of the particles inside the gas. The internal energy u of a thermodynamic system is the energy it contains. If work is done on the gas energy is added to the gas. The internal energy of a. Ideal Gas Internal Energy.
From www.researchgate.net
4 th order polynomial approximation to the NIST ideal gas internal Ideal Gas Internal Energy Internal energy of an ideal gas. At the same time, 140 j of energy is. The internal energy of an ideal gas consists of only kinetic energy. It can be due to the motion of its. The internal energy of a gas is defined to be the total energy of the gas when the center of mass of the gas. Ideal Gas Internal Energy.
From www.tec-science.com
Internal energy of ideal gases tecscience Ideal Gas Internal Energy A sample of an ideal gas in the cylinder of an engine is compressed from 400 ml to 50.0 ml during the compression stroke against a constant pressure of 8.00 atm. At the same time, 140 j of energy is. The internal energy of a gas is defined as: It can be due to the motion of its. The internal. Ideal Gas Internal Energy.
From www.slideserve.com
PPT Chapter 3 The First Law of Thermodynamics Closed System Ideal Gas Internal Energy In thermodynamics, the fact that the energy of an ideal gas depends only on temperature is an experimental observation from the free. The internal energy of an ideal gas consists of only kinetic energy. The internal energy u of a thermodynamic system is the energy it contains. The internal energy consists of the. Internal energy of an ideal gas. The. Ideal Gas Internal Energy.
From www.youtube.com
Internal energy & molar heat capacity of ideal gases (derivation Ideal Gas Internal Energy A sample of an ideal gas in the cylinder of an engine is compressed from 400 ml to 50.0 ml during the compression stroke against a constant pressure of 8.00 atm. At the same time, 140 j of energy is. The internal energy consists of the. The internal energy of a gas is defined to be the total energy of. Ideal Gas Internal Energy.
From www.youtube.com
11. Internal energy of an ideal gas YouTube Ideal Gas Internal Energy The internal energy of a gas is defined to be the total energy of the gas when the center of mass of the gas is at rest. The internal energy consists of the. Internal energy of an ideal gas. At the same time, 140 j of energy is. It can be due to the motion of its. The internal energy. Ideal Gas Internal Energy.
From www.youtube.com
The internal energy of an ideal diatomic gas corresponding to volume V Ideal Gas Internal Energy The internal energy u of a thermodynamic system is the energy it contains. Internal energy of an ideal gas. The internal energy of a gas is defined as: It can be due to the motion of its. The internal energy of a gas is defined to be the total energy of the gas when the center of mass of the. Ideal Gas Internal Energy.
From byjus.com
The internal energy of an ideal gas is sum of energy of Ideal Gas Internal Energy The internal energy of an ideal gas consists of only kinetic energy. The internal energy u of a thermodynamic system is the energy it contains. A sample of an ideal gas in the cylinder of an engine is compressed from 400 ml to 50.0 ml during the compression stroke against a constant pressure of 8.00 atm. It can be due. Ideal Gas Internal Energy.
From www.youtube.com
Example Using specific heat to calculate ideal gas internal energy Ideal Gas Internal Energy The internal energy of a gas is defined as: The internal energy of a gas is defined to be the total energy of the gas when the center of mass of the gas is at rest. The internal energy of an ideal gas consists of only kinetic energy. Internal energy of an ideal gas. The internal energy u of a. Ideal Gas Internal Energy.
From slideplayer.com
The Ideal Gas Law and Theory ppt download Ideal Gas Internal Energy Internal energy of an ideal gas. The internal energy u of a thermodynamic system is the energy it contains. Internal energy of an ideal gas. If work is done on the gas energy is added to the gas. The internal energy of a gas is defined to be the total energy of the gas when the center of mass of. Ideal Gas Internal Energy.
From mmerevise.co.uk
The Ideal Gas Equation MME Ideal Gas Internal Energy Internal energy of an ideal gas. The internal energy of a gas is defined as: It can be due to the motion of its. At the same time, 140 j of energy is. In thermodynamics, the fact that the energy of an ideal gas depends only on temperature is an experimental observation from the free. A sample of an ideal. Ideal Gas Internal Energy.
From www.youtube.com
Joule's free expansion, internal energy and enthalpy of an ideal gas Ideal Gas Internal Energy The sum of the kinetic and potential energies of the particles inside the gas. If work is done on the gas energy is added to the gas. A sample of an ideal gas in the cylinder of an engine is compressed from 400 ml to 50.0 ml during the compression stroke against a constant pressure of 8.00 atm. At the. Ideal Gas Internal Energy.
From www.slideserve.com
PPT Ideal gas PowerPoint Presentation, free download ID2134712 Ideal Gas Internal Energy The internal energy consists of the. The internal energy of an ideal gas consists of only kinetic energy. It can be due to the motion of its. A sample of an ideal gas in the cylinder of an engine is compressed from 400 ml to 50.0 ml during the compression stroke against a constant pressure of 8.00 atm. If work. Ideal Gas Internal Energy.
From www.slideserve.com
PPT C H A P T E R 14 The Ideal Gas Law and Theory PowerPoint Ideal Gas Internal Energy In thermodynamics, the fact that the energy of an ideal gas depends only on temperature is an experimental observation from the free. The sum of the kinetic and potential energies of the particles inside the gas. It can be due to the motion of its. The internal energy of a gas is defined to be the total energy of the. Ideal Gas Internal Energy.
From www.slideserve.com
PPT C H A P T E R 14 The Ideal Gas Law and Theory PowerPoint Ideal Gas Internal Energy It can be due to the motion of its. In thermodynamics, the fact that the energy of an ideal gas depends only on temperature is an experimental observation from the free. If work is done on the gas energy is added to the gas. A sample of an ideal gas in the cylinder of an engine is compressed from 400. Ideal Gas Internal Energy.
From www.reddit.com
Internal Energy of Ideal Gas System equation r/Mcat Ideal Gas Internal Energy Internal energy of an ideal gas. If work is done on the gas energy is added to the gas. The internal energy of a gas is defined as: The internal energy u of a thermodynamic system is the energy it contains. The internal energy consists of the. A sample of an ideal gas in the cylinder of an engine is. Ideal Gas Internal Energy.
From www.studocu.com
Basic Thermodynamics6 INTERNAL ENERGY AND ENTHALPY OF AN IDEAL GAS Ideal Gas Internal Energy A sample of an ideal gas in the cylinder of an engine is compressed from 400 ml to 50.0 ml during the compression stroke against a constant pressure of 8.00 atm. Internal energy of an ideal gas. It can be due to the motion of its. The internal energy u of a thermodynamic system is the energy it contains. The. Ideal Gas Internal Energy.
From www.slideserve.com
PPT BAB IV (CONT’D) PowerPoint Presentation, free download ID1318213 Ideal Gas Internal Energy In thermodynamics, the fact that the energy of an ideal gas depends only on temperature is an experimental observation from the free. The internal energy of a gas is defined to be the total energy of the gas when the center of mass of the gas is at rest. It can be due to the motion of its. A sample. Ideal Gas Internal Energy.
From www.youtube.com
Internal energy of an ideal gas depends upon YouTube Ideal Gas Internal Energy The internal energy consists of the. If work is done on the gas energy is added to the gas. The internal energy of an ideal gas consists of only kinetic energy. The internal energy of a gas is defined to be the total energy of the gas when the center of mass of the gas is at rest. It can. Ideal Gas Internal Energy.
From www.researchgate.net
4 th order polynomial approximation to the NIST ideal gas internal Ideal Gas Internal Energy The sum of the kinetic and potential energies of the particles inside the gas. The internal energy of an ideal gas consists of only kinetic energy. Internal energy of an ideal gas. The internal energy consists of the. If work is done on the gas energy is added to the gas. In thermodynamics, the fact that the energy of an. Ideal Gas Internal Energy.
From www.youtube.com
Internal Energy of Gas Thermodynamic Systems YouTube Ideal Gas Internal Energy The internal energy consists of the. Internal energy of an ideal gas. At the same time, 140 j of energy is. The internal energy of an ideal gas consists of only kinetic energy. The internal energy of a gas is defined to be the total energy of the gas when the center of mass of the gas is at rest.. Ideal Gas Internal Energy.
From www.slideserve.com
PPT Chapter 14Heat PowerPoint Presentation, free download ID4139496 Ideal Gas Internal Energy Internal energy of an ideal gas. The internal energy of a gas is defined as: It can be due to the motion of its. The sum of the kinetic and potential energies of the particles inside the gas. The internal energy consists of the. The internal energy u of a thermodynamic system is the energy it contains. Internal energy of. Ideal Gas Internal Energy.
From www.slideserve.com
PPT The Ideal Gas PowerPoint Presentation, free download ID6789672 Ideal Gas Internal Energy The internal energy of a gas is defined as: The sum of the kinetic and potential energies of the particles inside the gas. Internal energy of an ideal gas. The internal energy u of a thermodynamic system is the energy it contains. A sample of an ideal gas in the cylinder of an engine is compressed from 400 ml to. Ideal Gas Internal Energy.