Titration Lab Title . To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. This link makes a copy of the lab template that you use to develop your google lab. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Acid base titrations lab report links. The equivalence point is the point in a neutralization reaction where the number of moles of hydrogen ions is equal to the number of moles of hydroxide. Titrations can be used to determine unknown concentration of either analyte or titrate. In the titration, a specific volume of acidic solution is. Titrations are standard chemistry laboratory procedures usually used to determine the unknown concentration of a substance. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in.
from www.studocu.com
Acid base titrations lab report links. The equivalence point is the point in a neutralization reaction where the number of moles of hydrogen ions is equal to the number of moles of hydroxide. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. In the titration, a specific volume of acidic solution is. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. Titrations are standard chemistry laboratory procedures usually used to determine the unknown concentration of a substance. Titrations can be used to determine unknown concentration of either analyte or titrate. This link makes a copy of the lab template that you use to develop your google lab. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in.
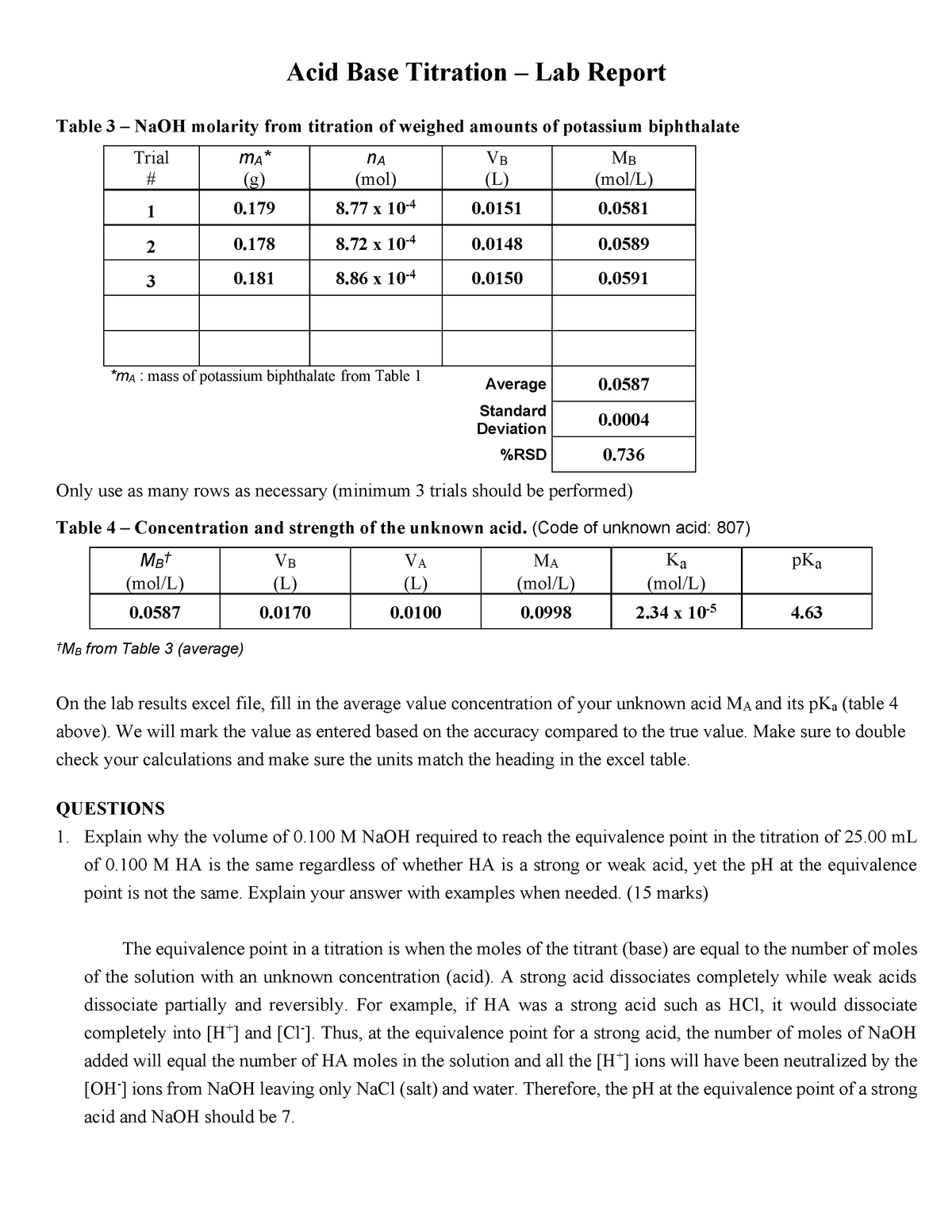
Titration Lab Report Acid Base Titration Lab Report Table 3 NaOH
Titration Lab Title In the titration, a specific volume of acidic solution is. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in. This link makes a copy of the lab template that you use to develop your google lab. Titrations are standard chemistry laboratory procedures usually used to determine the unknown concentration of a substance. The equivalence point is the point in a neutralization reaction where the number of moles of hydrogen ions is equal to the number of moles of hydroxide. Acid base titrations lab report links. Titrations can be used to determine unknown concentration of either analyte or titrate. In the titration, a specific volume of acidic solution is. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution.
From beyondlabz.freshdesk.com
Titrations Lab Overview Beyond Labz Titration Lab Title In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in. The equivalence point is the point in a neutralization reaction where the number of moles of hydrogen ions is equal to the number of moles of hydroxide. In the titration, a specific volume of. Titration Lab Title.
From www.scribd.com
Titration Lab Report PDF Titration Chemistry Titration Lab Title The equivalence point is the point in a neutralization reaction where the number of moles of hydrogen ions is equal to the number of moles of hydroxide. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in. Acid base titrations lab report links. Titrations. Titration Lab Title.
From www.studocu.com
Titration Lab Report Acid Base Titration Lab Report Table 3 NaOH Titration Lab Title This link makes a copy of the lab template that you use to develop your google lab. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in. The equivalence point is the point in a neutralization reaction where the number of moles of hydrogen. Titration Lab Title.
From joiyfxbtq.blob.core.windows.net
Titration Method Image at Jodie Massey blog Titration Lab Title To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. Titrations can be used to determine unknown concentration of either analyte or titrate. This link makes a copy of the lab template that you use to develop your google lab. The equivalence point is the point in a neutralization reaction where the number of moles of. Titration Lab Title.
From ar.inspiredpencil.com
Titration Pink Titration Lab Title In the titration, a specific volume of acidic solution is. Acid base titrations lab report links. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. This link makes a copy of the lab template that you use to develop your google lab. Titrations are standard chemistry laboratory. Titration Lab Title.
From mungfali.com
Acid Base Titration Procedure Titration Lab Title A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in. In the titration, a specific volume of acidic solution is. The equivalence. Titration Lab Title.
From exyobmsds.blob.core.windows.net
AcidBase Titration Lab Advanced Chemistry With Vernier Answers at Titration Lab Title A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. Acid base titrations lab report links. This link makes a copy of the lab template that you use to develop your google lab. Titrations. Titration Lab Title.
From celhcddi.blob.core.windows.net
Titration Lab Math at Mike Brown blog Titration Lab Title Acid base titrations lab report links. Titrations are standard chemistry laboratory procedures usually used to determine the unknown concentration of a substance. The equivalence point is the point in a neutralization reaction where the number of moles of hydrogen ions is equal to the number of moles of hydroxide. This link makes a copy of the lab template that you. Titration Lab Title.
From ceitiemh.blob.core.windows.net
Online Titration Lab Answers at Elsie Obrien blog Titration Lab Title This link makes a copy of the lab template that you use to develop your google lab. In the titration, a specific volume of acidic solution is. Titrations are standard chemistry laboratory procedures usually used to determine the unknown concentration of a substance. Acid base titrations lab report links. Titrations can be used to determine unknown concentration of either analyte. Titration Lab Title.
From dxoygshyx.blob.core.windows.net
Volumetric Titration Class 11 at Juana Nielsen blog Titration Lab Title The equivalence point is the point in a neutralization reaction where the number of moles of hydrogen ions is equal to the number of moles of hydroxide. In the titration, a specific volume of acidic solution is. Titrations are standard chemistry laboratory procedures usually used to determine the unknown concentration of a substance. A titration is an analytical procedure used. Titration Lab Title.
From melanie-chapter.blogspot.com
Titration Of Acids And Bases Lab Report Experiment 20 Answers 45+ Pages Titration Lab Title Titrations are standard chemistry laboratory procedures usually used to determine the unknown concentration of a substance. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. The equivalence point is the point in a neutralization reaction where the number of moles of hydrogen ions is equal to the number of moles of hydroxide. Acid base titrations. Titration Lab Title.
From vdocuments.site
Acid Base Titration Chalkbored Base Titration. Define Titration Lab Title This link makes a copy of the lab template that you use to develop your google lab. The equivalence point is the point in a neutralization reaction where the number of moles of hydrogen ions is equal to the number of moles of hydroxide. Titrations can be used to determine unknown concentration of either analyte or titrate. Titrations are standard. Titration Lab Title.
From studylib.net
Titration Lab Titration Lab Title The equivalence point is the point in a neutralization reaction where the number of moles of hydrogen ions is equal to the number of moles of hydroxide. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. This link makes a copy of the lab template that you use to develop your google lab. In a. Titration Lab Title.
From www.studocu.com
Titration Lab Report About the lab tiration Titration Lab Report Titration Lab Title A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. In the titration, a specific volume of acidic solution is. This link makes a copy of the lab template that you use to develop your google lab. Titrations are standard chemistry laboratory procedures usually used to determine the. Titration Lab Title.
From chem.libretexts.org
3.7 AcidBase Titrations Chemistry LibreTexts Titration Lab Title The equivalence point is the point in a neutralization reaction where the number of moles of hydrogen ions is equal to the number of moles of hydroxide. Titrations can be used to determine unknown concentration of either analyte or titrate. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a. Titration Lab Title.
From www.studocu.com
Titration Lab Sheet What does this tell you about the substance? it’s Titration Lab Title Titrations are standard chemistry laboratory procedures usually used to determine the unknown concentration of a substance. This link makes a copy of the lab template that you use to develop your google lab. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. In the titration, a specific. Titration Lab Title.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Lab Title Titrations can be used to determine unknown concentration of either analyte or titrate. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. Titration Lab Title.
From joieldmit.blob.core.windows.net
How Is Titration Used In Medicine at Shelia Wiley blog Titration Lab Title In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in. The equivalence point is the point in a neutralization reaction where the number of moles of hydrogen ions is equal to the number of moles of hydroxide. To find the concentration of sodium hydroxide. Titration Lab Title.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Lab Title To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. Titrations can be used to determine unknown concentration of either analyte or titrate. Titrations are standard chemistry laboratory procedures usually used to determine the unknown concentration of a substance. The equivalence point is the point in a neutralization reaction where the number of moles of hydrogen. Titration Lab Title.
From exokxncvu.blob.core.windows.net
Acid Base Titration Set Up at Eloy Owens blog Titration Lab Title Acid base titrations lab report links. This link makes a copy of the lab template that you use to develop your google lab. In the titration, a specific volume of acidic solution is. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. The equivalence point is the. Titration Lab Title.
From www.chegg.com
Solved Titration of Acid/BaseReport Form Your Name Title Titration Lab Title A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. The equivalence point is the point in a neutralization reaction where the number of moles of hydrogen ions is equal to the number of moles of hydroxide. In the titration, a specific volume of acidic solution is. Acid. Titration Lab Title.
From www.studocu.com
Lab 7 p H and titration lab report Name Titration Lab Title Titrations are standard chemistry laboratory procedures usually used to determine the unknown concentration of a substance. Titrations can be used to determine unknown concentration of either analyte or titrate. In the titration, a specific volume of acidic solution is. The equivalence point is the point in a neutralization reaction where the number of moles of hydrogen ions is equal to. Titration Lab Title.
From tuitiontube.com
Details about AcidBase Titration Lab in Easy Language Tuition Tube Titration Lab Title In the titration, a specific volume of acidic solution is. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. Titrations are standard chemistry laboratory procedures usually used to determine the unknown concentration of a substance. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a. Titration Lab Title.
From www.studocu.com
Titration 1 LABORATORY REPORT GENERAL CHEMISTRY Course Code CHM Titration Lab Title A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Titrations are standard chemistry laboratory procedures usually used to determine the unknown concentration of a substance. Acid base titrations lab report links. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. This link. Titration Lab Title.
From www.oercommons.org
General Chemistry for Health Sciences lab manual 10 Titration OER Titration Lab Title In the titration, a specific volume of acidic solution is. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. In a ph titration you measure the ph as a function of the volume. Titration Lab Title.
From www.studocu.com
Titration lab in chemistry Title Titration Lab Purpose/Introduction Titration Lab Title Titrations are standard chemistry laboratory procedures usually used to determine the unknown concentration of a substance. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point. Titration Lab Title.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration Lab Title Acid base titrations lab report links. This link makes a copy of the lab template that you use to develop your google lab. In the titration, a specific volume of acidic solution is. The equivalence point is the point in a neutralization reaction where the number of moles of hydrogen ions is equal to the number of moles of hydroxide.. Titration Lab Title.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Lab Title To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. Titrations can be used to determine unknown concentration of either analyte or titrate. Acid base titrations lab report links. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in. Titrations. Titration Lab Title.
From mungfali.com
Acid Base Titration Lab Titration Lab Title Titrations can be used to determine unknown concentration of either analyte or titrate. The equivalence point is the point in a neutralization reaction where the number of moles of hydrogen ions is equal to the number of moles of hydroxide. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a. Titration Lab Title.
From joizlgxlm.blob.core.windows.net
Titration Experiments In Supramolecular Chemistry at Larry Ferrill blog Titration Lab Title The equivalence point is the point in a neutralization reaction where the number of moles of hydrogen ions is equal to the number of moles of hydroxide. Titrations are standard chemistry laboratory procedures usually used to determine the unknown concentration of a substance. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. A titration is. Titration Lab Title.
From haileeqohicks.blogspot.com
Acid Base Titration Experiment Report HaileeqoHicks Titration Lab Title To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. Acid base titrations lab report links. Titrations can be used to determine unknown concentration of either analyte or titrate. In the titration, a specific volume of acidic solution is. In a ph titration you measure the ph as a function of the volume of titrant added. Titration Lab Title.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Titration Lab Title In the titration, a specific volume of acidic solution is. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in. The equivalence point is the point in a neutralization reaction where the number of moles of hydrogen ions is equal to the number of. Titration Lab Title.
From mungfali.com
Acid Base Titration Lab Titration Lab Title In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in. This link makes a copy of the lab template that you use to develop your google lab. The equivalence point is the point in a neutralization reaction where the number of moles of hydrogen. Titration Lab Title.
From www.studocu.com
Lab Back Titration N/A Topic Titrimetric Analysis Title Back Titration Lab Title This link makes a copy of the lab template that you use to develop your google lab. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. Titrations can be used to determine unknown. Titration Lab Title.
From www.scribd.com
LAB Report 3 Titration Chemistry Titration Lab Title The equivalence point is the point in a neutralization reaction where the number of moles of hydrogen ions is equal to the number of moles of hydroxide. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with. Titration Lab Title.