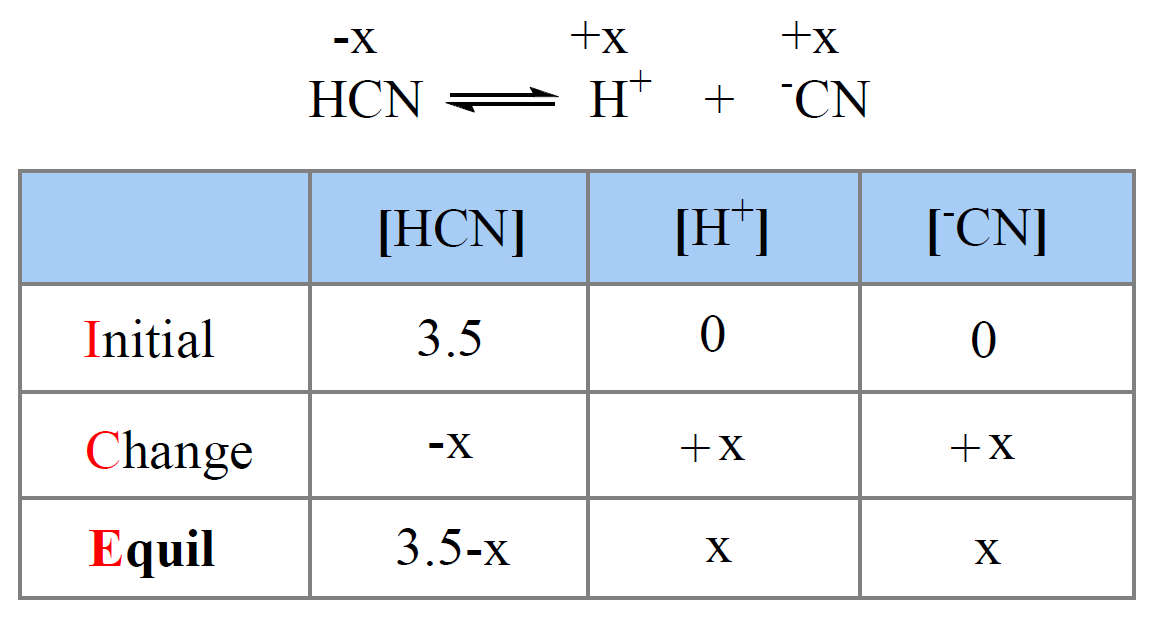
What Ph Is The Weakest Acid . At 25°c, \(pk_a + pk_b = 14.00\). This article shows simply the assumptions and the calculation methods for ka, ph, [h+] and [ha] for weak acids. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Calculate ph and poh of a weak acid or base solution using simple formula, quadratic. For example, concentrated vinegar (acetic acid, which is a weak acid) could have a lower ph than a dilute solution of hydrochloric acid (a strong acid). Ph of a weak acid. Learn how to calculate the ph of a weak acid with the help of step by step details and an example. On the other hand, the. Determining the ph of a weak acid is more complex compared to what we saw for strong acids, because for a weak acid, the dissociation is not 100%, and therefore, the. Learn more about the ph of acids, bases & more. Define a weak acid or base.
from general.chemistrysteps.com
On the other hand, the. Define a weak acid or base. Learn more about the ph of acids, bases & more. For example, concentrated vinegar (acetic acid, which is a weak acid) could have a lower ph than a dilute solution of hydrochloric acid (a strong acid). Calculate ph and poh of a weak acid or base solution using simple formula, quadratic. Learn how to calculate the ph of a weak acid with the help of step by step details and an example. Determining the ph of a weak acid is more complex compared to what we saw for strong acids, because for a weak acid, the dissociation is not 100%, and therefore, the. This article shows simply the assumptions and the calculation methods for ka, ph, [h+] and [ha] for weak acids. Ph of a weak acid. At 25°c, \(pk_a + pk_b = 14.00\).
pH of a Weak Acid Chemistry Steps
What Ph Is The Weakest Acid Ph of a weak acid. Calculate ph and poh of a weak acid or base solution using simple formula, quadratic. Define a weak acid or base. At 25°c, \(pk_a + pk_b = 14.00\). On the other hand, the. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Ph of a weak acid. Learn more about the ph of acids, bases & more. Determining the ph of a weak acid is more complex compared to what we saw for strong acids, because for a weak acid, the dissociation is not 100%, and therefore, the. This article shows simply the assumptions and the calculation methods for ka, ph, [h+] and [ha] for weak acids. Learn how to calculate the ph of a weak acid with the help of step by step details and an example. For example, concentrated vinegar (acetic acid, which is a weak acid) could have a lower ph than a dilute solution of hydrochloric acid (a strong acid).
From www.tes.com
pH of Weak Acids (A Level Chemistry) Teaching Resources What Ph Is The Weakest Acid For example, concentrated vinegar (acetic acid, which is a weak acid) could have a lower ph than a dilute solution of hydrochloric acid (a strong acid). Calculate ph and poh of a weak acid or base solution using simple formula, quadratic. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Ph of. What Ph Is The Weakest Acid.
From www.youtube.com
CHEMISTRY 201 Calculating the pH of a weak acid solution YouTube What Ph Is The Weakest Acid On the other hand, the. Determining the ph of a weak acid is more complex compared to what we saw for strong acids, because for a weak acid, the dissociation is not 100%, and therefore, the. Calculate ph and poh of a weak acid or base solution using simple formula, quadratic. Learn how to calculate the ph of a weak. What Ph Is The Weakest Acid.
From general.chemistrysteps.com
pH of a Weak Acid Chemistry Steps What Ph Is The Weakest Acid This article shows simply the assumptions and the calculation methods for ka, ph, [h+] and [ha] for weak acids. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Define a weak acid or base. For example, concentrated vinegar (acetic acid, which is a weak acid) could have a lower ph than a. What Ph Is The Weakest Acid.
From amchemistryblog.wordpress.com
4a. Acids and Bases Antonia's chemistry blog What Ph Is The Weakest Acid Learn how to calculate the ph of a weak acid with the help of step by step details and an example. Calculate ph and poh of a weak acid or base solution using simple formula, quadratic. Define a weak acid or base. Learn more about the ph of acids, bases & more. For example, concentrated vinegar (acetic acid, which is. What Ph Is The Weakest Acid.
From www.youtube.com
CHEMISTRY 201 Calculating Ka for a weak acid from pH YouTube What Ph Is The Weakest Acid Learn more about the ph of acids, bases & more. Learn how to calculate the ph of a weak acid with the help of step by step details and an example. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. On the other hand, the. Ph of a weak acid. This article. What Ph Is The Weakest Acid.
From slidesharetrick.blogspot.com
Ph Of Weak Acid And Weak Base Formula slidesharetrick What Ph Is The Weakest Acid Define a weak acid or base. On the other hand, the. Calculate ph and poh of a weak acid or base solution using simple formula, quadratic. Determining the ph of a weak acid is more complex compared to what we saw for strong acids, because for a weak acid, the dissociation is not 100%, and therefore, the. Learn more about. What Ph Is The Weakest Acid.
From www.slideserve.com
PPT Calculating the pH of Acids and Bases PowerPoint Presentation What Ph Is The Weakest Acid For example, concentrated vinegar (acetic acid, which is a weak acid) could have a lower ph than a dilute solution of hydrochloric acid (a strong acid). At 25°c, \(pk_a + pk_b = 14.00\). Ph of a weak acid. Define a weak acid or base. On the other hand, the. Learn how to calculate the ph of a weak acid with. What Ph Is The Weakest Acid.
From mavink.com
Ph Scale Acids And Bases What Ph Is The Weakest Acid The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. At 25°c, \(pk_a + pk_b = 14.00\). Ph of a weak acid. Calculate ph and poh of a weak acid or base solution using simple formula, quadratic. This article shows simply the assumptions and the calculation methods for ka, ph, [h+] and [ha]. What Ph Is The Weakest Acid.
From www.thoughtco.com
List of Common Strong and Weak Acids What Ph Is The Weakest Acid On the other hand, the. Determining the ph of a weak acid is more complex compared to what we saw for strong acids, because for a weak acid, the dissociation is not 100%, and therefore, the. Ph of a weak acid. This article shows simply the assumptions and the calculation methods for ka, ph, [h+] and [ha] for weak acids.. What Ph Is The Weakest Acid.
From www.teachoo.com
Examples of Weak Acids 5+ Examples Teachoo Teachoo Questions What Ph Is The Weakest Acid Determining the ph of a weak acid is more complex compared to what we saw for strong acids, because for a weak acid, the dissociation is not 100%, and therefore, the. Calculate ph and poh of a weak acid or base solution using simple formula, quadratic. On the other hand, the. The henderson hasselbalch equation finds the ph of a. What Ph Is The Weakest Acid.
From www.youtube.com
Calculating the pH of a Mixture of Weak Acids YouTube What Ph Is The Weakest Acid Learn more about the ph of acids, bases & more. For example, concentrated vinegar (acetic acid, which is a weak acid) could have a lower ph than a dilute solution of hydrochloric acid (a strong acid). Calculate ph and poh of a weak acid or base solution using simple formula, quadratic. Learn how to calculate the ph of a weak. What Ph Is The Weakest Acid.
From www.expii.com
The pH of Weak Acid and Base Solutions — Examples Expii What Ph Is The Weakest Acid Define a weak acid or base. Calculate ph and poh of a weak acid or base solution using simple formula, quadratic. For example, concentrated vinegar (acetic acid, which is a weak acid) could have a lower ph than a dilute solution of hydrochloric acid (a strong acid). At 25°c, \(pk_a + pk_b = 14.00\). This article shows simply the assumptions. What Ph Is The Weakest Acid.
From www.youtube.com
18.2 Calculating pH of weak acids and bases (HL) YouTube What Ph Is The Weakest Acid The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Define a weak acid or base. Learn how to calculate the ph of a weak acid with the help of step by step details and an example. Determining the ph of a weak acid is more complex compared to what we saw for. What Ph Is The Weakest Acid.
From general.chemistrysteps.com
pH of a Weak Acid Chemistry Steps What Ph Is The Weakest Acid Learn how to calculate the ph of a weak acid with the help of step by step details and an example. Calculate ph and poh of a weak acid or base solution using simple formula, quadratic. On the other hand, the. Determining the ph of a weak acid is more complex compared to what we saw for strong acids, because. What Ph Is The Weakest Acid.
From www.youtube.com
pH of Weak Acids and Bases Percent Ionization Ka & Kb YouTube What Ph Is The Weakest Acid This article shows simply the assumptions and the calculation methods for ka, ph, [h+] and [ha] for weak acids. Calculate ph and poh of a weak acid or base solution using simple formula, quadratic. On the other hand, the. Learn more about the ph of acids, bases & more. Ph of a weak acid. Determining the ph of a weak. What Ph Is The Weakest Acid.
From www.ck12.org
Acids and Bases CK12 Foundation What Ph Is The Weakest Acid Determining the ph of a weak acid is more complex compared to what we saw for strong acids, because for a weak acid, the dissociation is not 100%, and therefore, the. Learn more about the ph of acids, bases & more. Calculate ph and poh of a weak acid or base solution using simple formula, quadratic. At 25°c, \(pk_a +. What Ph Is The Weakest Acid.
From www.sciencelearn.org.nz
pH scale — Science Learning Hub What Ph Is The Weakest Acid On the other hand, the. Define a weak acid or base. Determining the ph of a weak acid is more complex compared to what we saw for strong acids, because for a weak acid, the dissociation is not 100%, and therefore, the. Learn more about the ph of acids, bases & more. Learn how to calculate the ph of a. What Ph Is The Weakest Acid.
From secondaryscience4all.wordpress.com
Acids and bases Secondary Science 4 All What Ph Is The Weakest Acid This article shows simply the assumptions and the calculation methods for ka, ph, [h+] and [ha] for weak acids. Learn more about the ph of acids, bases & more. Define a weak acid or base. Ph of a weak acid. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. At 25°c, \(pk_a. What Ph Is The Weakest Acid.
From www.alamy.com
Diagram of the pH scale with examples of acidic, neutral and alkaline What Ph Is The Weakest Acid Calculate ph and poh of a weak acid or base solution using simple formula, quadratic. Define a weak acid or base. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. For example, concentrated vinegar (acetic acid, which is a weak acid) could have a lower ph than a dilute solution of hydrochloric. What Ph Is The Weakest Acid.
From www.slideserve.com
PPT Unit 9 Notes PowerPoint Presentation, free download ID5460662 What Ph Is The Weakest Acid The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. For example, concentrated vinegar (acetic acid, which is a weak acid) could have a lower ph than a dilute solution of hydrochloric acid (a strong acid). Calculate ph and poh of a weak acid or base solution using simple formula, quadratic. Learn how. What Ph Is The Weakest Acid.
From www.sliderbase.com
Acids, pH and Equilibrium Presentation Chemistry What Ph Is The Weakest Acid Ph of a weak acid. Learn more about the ph of acids, bases & more. This article shows simply the assumptions and the calculation methods for ka, ph, [h+] and [ha] for weak acids. Define a weak acid or base. Calculate ph and poh of a weak acid or base solution using simple formula, quadratic. Determining the ph of a. What Ph Is The Weakest Acid.
From skc13chem.blogspot.com
SKC year 13 Chemistry calculating pH of a weak acid What Ph Is The Weakest Acid For example, concentrated vinegar (acetic acid, which is a weak acid) could have a lower ph than a dilute solution of hydrochloric acid (a strong acid). On the other hand, the. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Calculate ph and poh of a weak acid or base solution using. What Ph Is The Weakest Acid.
From alevelchemistry.co.uk
Acids Facts, Summary, Weak & Strong ALevel Chemistry Revision What Ph Is The Weakest Acid Determining the ph of a weak acid is more complex compared to what we saw for strong acids, because for a weak acid, the dissociation is not 100%, and therefore, the. Define a weak acid or base. Learn more about the ph of acids, bases & more. Learn how to calculate the ph of a weak acid with the help. What Ph Is The Weakest Acid.
From www.slideserve.com
PPT The Equilibrium of Weak Acids and Bases PowerPoint Presentation What Ph Is The Weakest Acid The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. For example, concentrated vinegar (acetic acid, which is a weak acid) could have a lower ph than a dilute solution of hydrochloric acid (a strong acid). Learn more about the ph of acids, bases & more. Learn how to calculate the ph of. What Ph Is The Weakest Acid.
From techiescientist.com
pH of Lactic Acid — Strong or Weak? Techiescientist What Ph Is The Weakest Acid This article shows simply the assumptions and the calculation methods for ka, ph, [h+] and [ha] for weak acids. Determining the ph of a weak acid is more complex compared to what we saw for strong acids, because for a weak acid, the dissociation is not 100%, and therefore, the. On the other hand, the. The henderson hasselbalch equation finds. What Ph Is The Weakest Acid.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download What Ph Is The Weakest Acid This article shows simply the assumptions and the calculation methods for ka, ph, [h+] and [ha] for weak acids. Determining the ph of a weak acid is more complex compared to what we saw for strong acids, because for a weak acid, the dissociation is not 100%, and therefore, the. For example, concentrated vinegar (acetic acid, which is a weak. What Ph Is The Weakest Acid.
From www.youtube.com
Calculating pH for weak acids YouTube What Ph Is The Weakest Acid Determining the ph of a weak acid is more complex compared to what we saw for strong acids, because for a weak acid, the dissociation is not 100%, and therefore, the. Define a weak acid or base. At 25°c, \(pk_a + pk_b = 14.00\). The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak. What Ph Is The Weakest Acid.
From acidsandbasesrios.weebly.com
pH Acids and Bases What Ph Is The Weakest Acid Determining the ph of a weak acid is more complex compared to what we saw for strong acids, because for a weak acid, the dissociation is not 100%, and therefore, the. Learn more about the ph of acids, bases & more. On the other hand, the. Learn how to calculate the ph of a weak acid with the help of. What Ph Is The Weakest Acid.
From www.slideserve.com
PPT Finding the pH of Weak Acids PowerPoint Presentation, free What Ph Is The Weakest Acid This article shows simply the assumptions and the calculation methods for ka, ph, [h+] and [ha] for weak acids. At 25°c, \(pk_a + pk_b = 14.00\). Learn how to calculate the ph of a weak acid with the help of step by step details and an example. Learn more about the ph of acids, bases & more. Determining the ph. What Ph Is The Weakest Acid.
From preclaboratories.com
Back to Basics Acids, Bases & the pH Scale Precision Laboratories What Ph Is The Weakest Acid Ph of a weak acid. Determining the ph of a weak acid is more complex compared to what we saw for strong acids, because for a weak acid, the dissociation is not 100%, and therefore, the. Learn more about the ph of acids, bases & more. On the other hand, the. Calculate ph and poh of a weak acid or. What Ph Is The Weakest Acid.
From www.slideserve.com
PPT Weak and Strong Acids PowerPoint Presentation, free download ID What Ph Is The Weakest Acid Define a weak acid or base. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Determining the ph of a weak acid is more complex compared to what we saw for strong acids, because for a weak acid, the dissociation is not 100%, and therefore, the. Learn how to calculate the ph. What Ph Is The Weakest Acid.
From www.teachoo.com
Strength of Acids and Bases (How to find it?) Chemistry Teachoo What Ph Is The Weakest Acid This article shows simply the assumptions and the calculation methods for ka, ph, [h+] and [ha] for weak acids. Ph of a weak acid. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Calculate ph and poh of a weak acid or base solution using simple formula, quadratic. Learn how to calculate. What Ph Is The Weakest Acid.
From www.slideserve.com
PPT Finding the pH of Weak Acids PowerPoint Presentation, free What Ph Is The Weakest Acid On the other hand, the. Determining the ph of a weak acid is more complex compared to what we saw for strong acids, because for a weak acid, the dissociation is not 100%, and therefore, the. Learn how to calculate the ph of a weak acid with the help of step by step details and an example. Define a weak. What Ph Is The Weakest Acid.
From www.slideserve.com
PPT Reactions of acids and bases… PowerPoint Presentation, free What Ph Is The Weakest Acid Learn how to calculate the ph of a weak acid with the help of step by step details and an example. On the other hand, the. At 25°c, \(pk_a + pk_b = 14.00\). This article shows simply the assumptions and the calculation methods for ka, ph, [h+] and [ha] for weak acids. Calculate ph and poh of a weak acid. What Ph Is The Weakest Acid.
From www.slideserve.com
PPT Calculating the pH of Acids and Bases PowerPoint Presentation What Ph Is The Weakest Acid Ph of a weak acid. Learn how to calculate the ph of a weak acid with the help of step by step details and an example. At 25°c, \(pk_a + pk_b = 14.00\). The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Learn more about the ph of acids, bases & more.. What Ph Is The Weakest Acid.