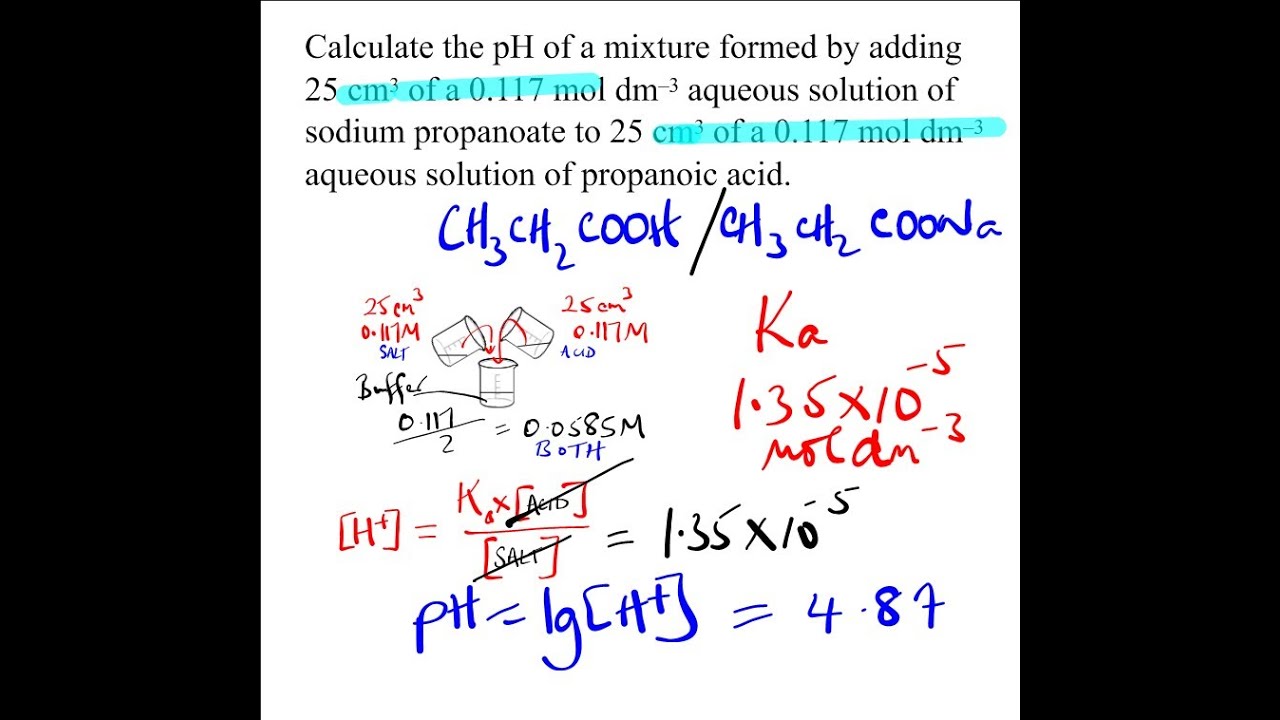
Buffer Solution Problems And Answers Pdf . A buffer solution is prepared by dissolving 1.51 g of nh3 and 3.85 g. If acid is added to the solution, it is consumed by the. Why are the effects not the same? A mixture of hno2(aq) and nano2(aq) is a better buffer because it consists of a weak acid (hno2) and its conjugate base. Use the titrations on the web site (move the slider bar to. In everyday english, a buffer is something that lessens the impact of an external force. This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. Three different buffer solutions are prepared in the laboratory that contain mixtures of hf(aq) and naf(aq). A buffer consists of a weak acid and its conjugate base in roughly equal amounts. A particulate representation of a small.
from criticalthinking.cloud
In everyday english, a buffer is something that lessens the impact of an external force. A particulate representation of a small. A buffer solution is prepared by dissolving 1.51 g of nh3 and 3.85 g. This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. Use the titrations on the web site (move the slider bar to. Three different buffer solutions are prepared in the laboratory that contain mixtures of hf(aq) and naf(aq). A mixture of hno2(aq) and nano2(aq) is a better buffer because it consists of a weak acid (hno2) and its conjugate base. A buffer consists of a weak acid and its conjugate base in roughly equal amounts. If acid is added to the solution, it is consumed by the. Why are the effects not the same?
buffer solution questions a level chemistry
Buffer Solution Problems And Answers Pdf Use the titrations on the web site (move the slider bar to. A buffer solution is prepared by dissolving 1.51 g of nh3 and 3.85 g. A buffer consists of a weak acid and its conjugate base in roughly equal amounts. A mixture of hno2(aq) and nano2(aq) is a better buffer because it consists of a weak acid (hno2) and its conjugate base. In everyday english, a buffer is something that lessens the impact of an external force. This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. A particulate representation of a small. Why are the effects not the same? Use the titrations on the web site (move the slider bar to. Three different buffer solutions are prepared in the laboratory that contain mixtures of hf(aq) and naf(aq). If acid is added to the solution, it is consumed by the.
From www.formsbank.com
Buffer Solutions Worksheet With Answers printable pdf download Buffer Solution Problems And Answers Pdf Why are the effects not the same? A mixture of hno2(aq) and nano2(aq) is a better buffer because it consists of a weak acid (hno2) and its conjugate base. Use the titrations on the web site (move the slider bar to. A particulate representation of a small. Three different buffer solutions are prepared in the laboratory that contain mixtures of. Buffer Solution Problems And Answers Pdf.
From www.pinterest.es
Understanding Buffer Solution Chemistry A Complete Guide Buffer Solution Problems And Answers Pdf In everyday english, a buffer is something that lessens the impact of an external force. A mixture of hno2(aq) and nano2(aq) is a better buffer because it consists of a weak acid (hno2) and its conjugate base. If acid is added to the solution, it is consumed by the. A particulate representation of a small. This is a summary practice. Buffer Solution Problems And Answers Pdf.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer Solution Problems And Answers Pdf A mixture of hno2(aq) and nano2(aq) is a better buffer because it consists of a weak acid (hno2) and its conjugate base. A buffer consists of a weak acid and its conjugate base in roughly equal amounts. Use the titrations on the web site (move the slider bar to. If acid is added to the solution, it is consumed by. Buffer Solution Problems And Answers Pdf.
From www.youtube.com
17.2 Buffer Example Problem YouTube Buffer Solution Problems And Answers Pdf A buffer solution is prepared by dissolving 1.51 g of nh3 and 3.85 g. Why are the effects not the same? If acid is added to the solution, it is consumed by the. This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. A buffer consists of. Buffer Solution Problems And Answers Pdf.
From www.slideserve.com
PPT Experiment 7 Preparation and Properties of Buffers PowerPoint Buffer Solution Problems And Answers Pdf A mixture of hno2(aq) and nano2(aq) is a better buffer because it consists of a weak acid (hno2) and its conjugate base. Three different buffer solutions are prepared in the laboratory that contain mixtures of hf(aq) and naf(aq). This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution. Buffer Solution Problems And Answers Pdf.
From www.ck12.org
Buffer Solutions Example 2 ( Video ) Chemistry CK12 Foundation Buffer Solution Problems And Answers Pdf A buffer solution is prepared by dissolving 1.51 g of nh3 and 3.85 g. Three different buffer solutions are prepared in the laboratory that contain mixtures of hf(aq) and naf(aq). In everyday english, a buffer is something that lessens the impact of an external force. A buffer consists of a weak acid and its conjugate base in roughly equal amounts.. Buffer Solution Problems And Answers Pdf.
From kunduz.com
[ANSWERED] Which of the following is a buffer solution Question Type Buffer Solution Problems And Answers Pdf In everyday english, a buffer is something that lessens the impact of an external force. A buffer solution is prepared by dissolving 1.51 g of nh3 and 3.85 g. Three different buffer solutions are prepared in the laboratory that contain mixtures of hf(aq) and naf(aq). A particulate representation of a small. Use the titrations on the web site (move the. Buffer Solution Problems And Answers Pdf.
From www.slideserve.com
PPT Ch. 16 Ionic Equilibria PowerPoint Presentation, free download Buffer Solution Problems And Answers Pdf A mixture of hno2(aq) and nano2(aq) is a better buffer because it consists of a weak acid (hno2) and its conjugate base. In everyday english, a buffer is something that lessens the impact of an external force. Use the titrations on the web site (move the slider bar to. Three different buffer solutions are prepared in the laboratory that contain. Buffer Solution Problems And Answers Pdf.
From www.pinterest.com
Pin on School Buffer Solution Problems And Answers Pdf Use the titrations on the web site (move the slider bar to. A buffer solution is prepared by dissolving 1.51 g of nh3 and 3.85 g. This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. A mixture of hno2(aq) and nano2(aq) is a better buffer because. Buffer Solution Problems And Answers Pdf.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation Buffer Solution Problems And Answers Pdf If acid is added to the solution, it is consumed by the. Three different buffer solutions are prepared in the laboratory that contain mixtures of hf(aq) and naf(aq). In everyday english, a buffer is something that lessens the impact of an external force. A particulate representation of a small. Use the titrations on the web site (move the slider bar. Buffer Solution Problems And Answers Pdf.
From studylib.net
Chemguide answers BUFFER SOLUTIONS Buffer Solution Problems And Answers Pdf A buffer solution is prepared by dissolving 1.51 g of nh3 and 3.85 g. If acid is added to the solution, it is consumed by the. A mixture of hno2(aq) and nano2(aq) is a better buffer because it consists of a weak acid (hno2) and its conjugate base. Three different buffer solutions are prepared in the laboratory that contain mixtures. Buffer Solution Problems And Answers Pdf.
From studylib.net
Buffer Solutions Buffer Solution Problems And Answers Pdf A mixture of hno2(aq) and nano2(aq) is a better buffer because it consists of a weak acid (hno2) and its conjugate base. Use the titrations on the web site (move the slider bar to. If acid is added to the solution, it is consumed by the. A buffer solution is prepared by dissolving 1.51 g of nh3 and 3.85 g.. Buffer Solution Problems And Answers Pdf.
From www.formsbank.com
Buffers Worksheet With Answers printable pdf download Buffer Solution Problems And Answers Pdf A buffer consists of a weak acid and its conjugate base in roughly equal amounts. If acid is added to the solution, it is consumed by the. In everyday english, a buffer is something that lessens the impact of an external force. Why are the effects not the same? A particulate representation of a small. Three different buffer solutions are. Buffer Solution Problems And Answers Pdf.
From morioh.com
Buffer Solutions Buffer Solution Problems And Answers Pdf A particulate representation of a small. Why are the effects not the same? This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. A buffer consists of a weak acid and its conjugate base in roughly equal amounts. If acid is added to the solution, it is. Buffer Solution Problems And Answers Pdf.
From www.studocu.com
Buffer PracticeKey Practice Worksheet key Buffer Practice Problems Buffer Solution Problems And Answers Pdf Why are the effects not the same? A mixture of hno2(aq) and nano2(aq) is a better buffer because it consists of a weak acid (hno2) and its conjugate base. A buffer solution is prepared by dissolving 1.51 g of nh3 and 3.85 g. Three different buffer solutions are prepared in the laboratory that contain mixtures of hf(aq) and naf(aq). A. Buffer Solution Problems And Answers Pdf.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer Solution Problems And Answers Pdf A particulate representation of a small. Use the titrations on the web site (move the slider bar to. Why are the effects not the same? In everyday english, a buffer is something that lessens the impact of an external force. A buffer consists of a weak acid and its conjugate base in roughly equal amounts. A buffer solution is prepared. Buffer Solution Problems And Answers Pdf.
From www.artofit.org
Buffer solutions examples and buffer chemistry notes Artofit Buffer Solution Problems And Answers Pdf Why are the effects not the same? Use the titrations on the web site (move the slider bar to. Three different buffer solutions are prepared in the laboratory that contain mixtures of hf(aq) and naf(aq). If acid is added to the solution, it is consumed by the. This is a summary practice problem set on buffer solutions aimed to help. Buffer Solution Problems And Answers Pdf.
From pharmacyscope.com
What is the Buffer Solution? Definition and ph of buffer solution Buffer Solution Problems And Answers Pdf Why are the effects not the same? Use the titrations on the web site (move the slider bar to. A buffer solution is prepared by dissolving 1.51 g of nh3 and 3.85 g. A buffer consists of a weak acid and its conjugate base in roughly equal amounts. In everyday english, a buffer is something that lessens the impact of. Buffer Solution Problems And Answers Pdf.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Buffer Solution Problems And Answers Pdf Use the titrations on the web site (move the slider bar to. If acid is added to the solution, it is consumed by the. A buffer consists of a weak acid and its conjugate base in roughly equal amounts. A buffer solution is prepared by dissolving 1.51 g of nh3 and 3.85 g. A mixture of hno2(aq) and nano2(aq) is. Buffer Solution Problems And Answers Pdf.
From www.pdfprof.com
application of buffer solution Buffer Solution Problems And Answers Pdf A buffer consists of a weak acid and its conjugate base in roughly equal amounts. If acid is added to the solution, it is consumed by the. Use the titrations on the web site (move the slider bar to. This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer. Buffer Solution Problems And Answers Pdf.
From criticalthinking.cloud
how to solve buffer problems chemistry Buffer Solution Problems And Answers Pdf If acid is added to the solution, it is consumed by the. In everyday english, a buffer is something that lessens the impact of an external force. Use the titrations on the web site (move the slider bar to. A particulate representation of a small. A buffer consists of a weak acid and its conjugate base in roughly equal amounts.. Buffer Solution Problems And Answers Pdf.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer Solution Problems And Answers Pdf Use the titrations on the web site (move the slider bar to. A buffer solution is prepared by dissolving 1.51 g of nh3 and 3.85 g. A particulate representation of a small. A buffer consists of a weak acid and its conjugate base in roughly equal amounts. In everyday english, a buffer is something that lessens the impact of an. Buffer Solution Problems And Answers Pdf.
From www.scribd.com
AP Unit9 Worksheet Answers Buffer Solution Acid Buffer Solution Problems And Answers Pdf In everyday english, a buffer is something that lessens the impact of an external force. A mixture of hno2(aq) and nano2(aq) is a better buffer because it consists of a weak acid (hno2) and its conjugate base. Why are the effects not the same? If acid is added to the solution, it is consumed by the. Use the titrations on. Buffer Solution Problems And Answers Pdf.
From criticalthinking.cloud
buffer solution questions a level chemistry Buffer Solution Problems And Answers Pdf A mixture of hno2(aq) and nano2(aq) is a better buffer because it consists of a weak acid (hno2) and its conjugate base. Three different buffer solutions are prepared in the laboratory that contain mixtures of hf(aq) and naf(aq). A buffer consists of a weak acid and its conjugate base in roughly equal amounts. This is a summary practice problem set. Buffer Solution Problems And Answers Pdf.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk Buffer Solution Problems And Answers Pdf A mixture of hno2(aq) and nano2(aq) is a better buffer because it consists of a weak acid (hno2) and its conjugate base. If acid is added to the solution, it is consumed by the. A buffer consists of a weak acid and its conjugate base in roughly equal amounts. In everyday english, a buffer is something that lessens the impact. Buffer Solution Problems And Answers Pdf.
From www.lessonplanet.com
Buffer Solution Problems Worksheet for 10th Higher Ed Lesson Buffer Solution Problems And Answers Pdf A mixture of hno2(aq) and nano2(aq) is a better buffer because it consists of a weak acid (hno2) and its conjugate base. If acid is added to the solution, it is consumed by the. Three different buffer solutions are prepared in the laboratory that contain mixtures of hf(aq) and naf(aq). Why are the effects not the same? Use the titrations. Buffer Solution Problems And Answers Pdf.
From www.academia.edu
(PDF) Buffer solutions hanul kim Academia.edu Buffer Solution Problems And Answers Pdf In everyday english, a buffer is something that lessens the impact of an external force. If acid is added to the solution, it is consumed by the. A buffer solution is prepared by dissolving 1.51 g of nh3 and 3.85 g. A particulate representation of a small. Three different buffer solutions are prepared in the laboratory that contain mixtures of. Buffer Solution Problems And Answers Pdf.
From www.studocu.com
Worksheet 6 buffer problems Worksheet 6 Buffer Solution Problems Buffer Solution Problems And Answers Pdf A buffer consists of a weak acid and its conjugate base in roughly equal amounts. A particulate representation of a small. Why are the effects not the same? If acid is added to the solution, it is consumed by the. Three different buffer solutions are prepared in the laboratory that contain mixtures of hf(aq) and naf(aq). A mixture of hno2(aq). Buffer Solution Problems And Answers Pdf.
From www.vrogue.co
Solved 1 Of 7 Chem 130 Making A Buffer Solution Name vrogue.co Buffer Solution Problems And Answers Pdf A buffer solution is prepared by dissolving 1.51 g of nh3 and 3.85 g. A particulate representation of a small. Use the titrations on the web site (move the slider bar to. This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. If acid is added to. Buffer Solution Problems And Answers Pdf.
From criticalthinking.cloud
buffer solution questions a level chemistry Buffer Solution Problems And Answers Pdf This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. A particulate representation of a small. Use the titrations on the web site (move the slider bar to. A mixture of hno2(aq) and nano2(aq) is a better buffer because it consists of a weak acid (hno2) and. Buffer Solution Problems And Answers Pdf.
From www.youtube.com
Buffer solutions 10, lecture 2 YouTube Buffer Solution Problems And Answers Pdf If acid is added to the solution, it is consumed by the. A particulate representation of a small. A buffer solution is prepared by dissolving 1.51 g of nh3 and 3.85 g. A mixture of hno2(aq) and nano2(aq) is a better buffer because it consists of a weak acid (hno2) and its conjugate base. This is a summary practice problem. Buffer Solution Problems And Answers Pdf.
From www.aakash.ac.in
Acidic Buffer Solution Definition, Calculation & Applications Buffer Solution Problems And Answers Pdf A buffer consists of a weak acid and its conjugate base in roughly equal amounts. This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. A buffer solution is prepared by dissolving 1.51 g of nh3 and 3.85 g. Three different buffer solutions are prepared in the. Buffer Solution Problems And Answers Pdf.
From www.researchgate.net
(PDF) Buffer solutions Buffer Solution Problems And Answers Pdf If acid is added to the solution, it is consumed by the. A buffer consists of a weak acid and its conjugate base in roughly equal amounts. Why are the effects not the same? Three different buffer solutions are prepared in the laboratory that contain mixtures of hf(aq) and naf(aq). A buffer solution is prepared by dissolving 1.51 g of. Buffer Solution Problems And Answers Pdf.
From studylib.net
Buffer Problems with answers Buffer Solution Problems And Answers Pdf This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. A mixture of hno2(aq) and nano2(aq) is a better buffer because it consists of a weak acid (hno2) and its conjugate base. Three different buffer solutions are prepared in the laboratory that contain mixtures of hf(aq) and. Buffer Solution Problems And Answers Pdf.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer Solution Problems And Answers Pdf In everyday english, a buffer is something that lessens the impact of an external force. If acid is added to the solution, it is consumed by the. Three different buffer solutions are prepared in the laboratory that contain mixtures of hf(aq) and naf(aq). A mixture of hno2(aq) and nano2(aq) is a better buffer because it consists of a weak acid. Buffer Solution Problems And Answers Pdf.