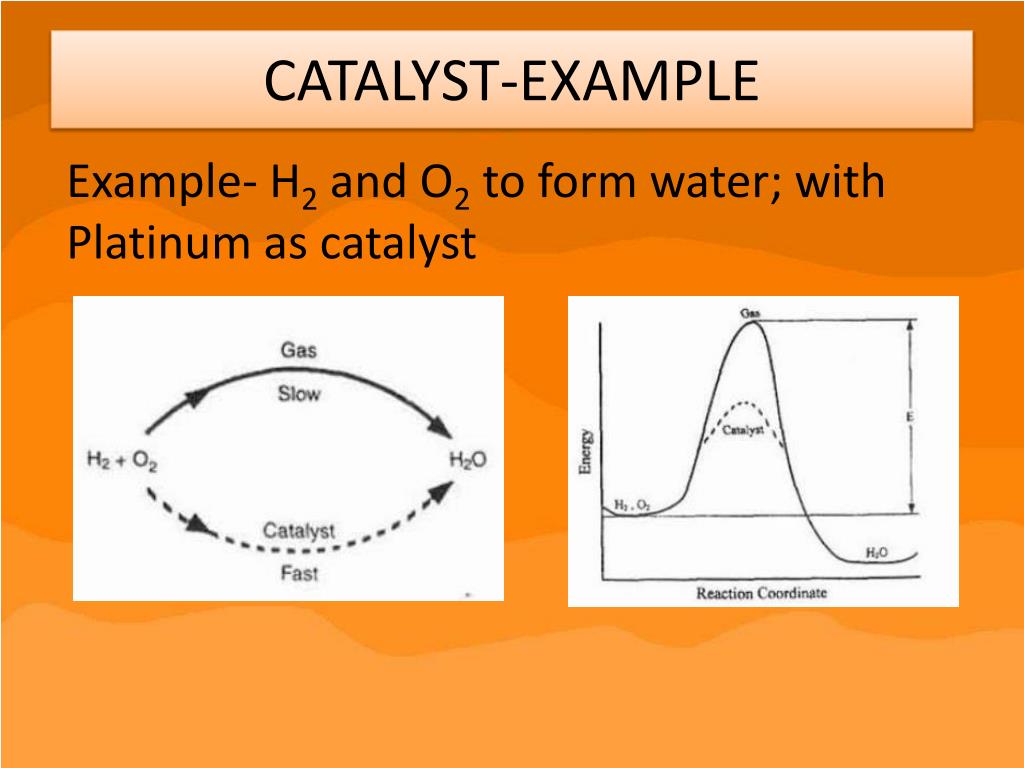
Catalyst Definition Physical Science . A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalysts work by providing an. A catalyst is a substance that increases the rate of a chemical reaction without being consumed or permanently altered by the reaction itself. A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; Industrial applications of catalysts include. They only help achieve equilibrium faster. A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is some material that speeds up chemical reactions. Hence a catalyst can be recovered chemically unchanged at the end of the reaction it has. Catalysts do not alter the equilibrium position of a reaction; With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds.
from www.slideserve.com
Industrial applications of catalysts include. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts work by providing an. A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. Hence a catalyst can be recovered chemically unchanged at the end of the reaction it has. A catalyst is a substance that increases the rate of a chemical reaction without being consumed or permanently altered by the reaction itself. Catalysts do not alter the equilibrium position of a reaction; They only help achieve equilibrium faster.
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint
Catalyst Definition Physical Science Industrial applications of catalysts include. They only help achieve equilibrium faster. A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Catalysts work by providing an. Catalysts do not alter the equilibrium position of a reaction; Hence a catalyst can be recovered chemically unchanged at the end of the reaction it has. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Industrial applications of catalysts include. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that increases the rate of a chemical reaction without being consumed or permanently altered by the reaction itself. A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; A catalyst is some material that speeds up chemical reactions.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation Catalyst Definition Physical Science They only help achieve equilibrium faster. Catalysts do not alter the equilibrium position of a reaction; Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that increases the rate of a chemical reaction without being consumed or permanently altered by the reaction itself. Catalysts work by providing an.. Catalyst Definition Physical Science.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyst Definition Physical Science Industrial applications of catalysts include. A catalyst is a substance that increases the rate of a chemical reaction without being consumed or permanently altered by the reaction itself. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Catalysts do not alter the equilibrium position of a reaction; Catalysts work. Catalyst Definition Physical Science.
From gamesmartz.com
Heterogeneous Catalyst Definition & Image GameSmartz Catalyst Definition Physical Science Hence a catalyst can be recovered chemically unchanged at the end of the reaction it has. A catalyst is some material that speeds up chemical reactions. Catalysts do not alter the equilibrium position of a reaction; Industrial applications of catalysts include. A catalyst is a substance that increases the rate of a chemical reaction without being consumed or permanently altered. Catalyst Definition Physical Science.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst Definition Physical Science A catalyst is a substance that increases the rate of a chemical reaction without being consumed or permanently altered by the reaction itself. A catalyst is some material that speeds up chemical reactions. Hence a catalyst can be recovered chemically unchanged at the end of the reaction it has. Industrial applications of catalysts include. A catalyst is a substance that. Catalyst Definition Physical Science.
From ar.inspiredpencil.com
Catalyst Chemistry Catalyst Definition Physical Science They only help achieve equilibrium faster. A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. A catalyst is a substance that increases the rate of a chemical reaction without being consumed or permanently altered by the reaction itself. A catalyst is a substance that speeds up a chemical reaction, but. Catalyst Definition Physical Science.
From giojfattf.blob.core.windows.net
Catalysis Definition Simple at Frederick Helm blog Catalyst Definition Physical Science Catalysts do not alter the equilibrium position of a reaction; With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. Catalyst, in chemistry, any substance that increases the rate of a. Catalyst Definition Physical Science.
From ar.inspiredpencil.com
Catalyst Catalyst Definition Physical Science With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalysts work by providing an. They only help achieve equilibrium faster. Catalysts do not alter. Catalyst Definition Physical Science.
From study.com
Catalysts Definition, Types & Examples Video & Lesson Transcript Catalyst Definition Physical Science A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst is a substance that increases the rate of a chemical reaction without being consumed or permanently altered by the reaction itself. With a helping hand from a catalyst, molecules that might take years. Catalyst Definition Physical Science.
From www.pinterest.com.mx
Catalysis Chemistry classroom, Chemistry Catalyst Definition Physical Science With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Catalysts work by providing an. Hence a catalyst can be recovered chemically unchanged at the end of the reaction it has. They only help achieve equilibrium faster. A catalyst is a substance that increases the rate of a chemical reaction. Catalyst Definition Physical Science.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Definition Physical Science A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. With a helping hand from a catalyst, molecules that might take years to interact can now do. Catalyst Definition Physical Science.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation, free download ID1803655 Catalyst Definition Physical Science A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Hence a catalyst. Catalyst Definition Physical Science.
From www.slideserve.com
PPT Reaction Rate PowerPoint Presentation, free download ID3534068 Catalyst Definition Physical Science Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Hence a catalyst can be recovered chemically unchanged at the end of the reaction it has. A catalyst is a substance that increases the rate of a chemical reaction without being consumed or permanently altered by the reaction itself. Catalysts work by providing an.. Catalyst Definition Physical Science.
From www.slideshare.net
Biology 2.4 Catalyst Definition Physical Science Catalysts do not alter the equilibrium position of a reaction; They only help achieve equilibrium faster. A catalyst is some material that speeds up chemical reactions. A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. Industrial applications of catalysts include. A catalyst is a substance that increases the rate of. Catalyst Definition Physical Science.
From www.youtube.com
Catalysis & Catalyst Definition & Types of catalysis Surface Chemistry Catalyst Definition Physical Science A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. A catalyst is a substance that increases the rate of a chemical reaction without being consumed or permanently altered by the reaction itself. Catalysts work by providing an. Hence a catalyst can be recovered chemically unchanged at the end of the. Catalyst Definition Physical Science.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica Catalyst Definition Physical Science With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Industrial applications of catalysts include. A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the. Catalyst Definition Physical Science.
From scienceinfo.com
Catalysis Definition, Types, Advantages, Disadvantages Catalyst Definition Physical Science A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that increases the rate of a chemical reaction without being consumed or permanently altered. Catalyst Definition Physical Science.
From www.mdpi.com
Catalysts Free FullText Heterogeneous Photocatalysis Recent Catalyst Definition Physical Science They only help achieve equilibrium faster. A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. Catalysts do not alter the equilibrium position of a reaction; Hence a catalyst can be recovered chemically unchanged at the end of the reaction it has. A catalyst is a substance that speeds up a. Catalyst Definition Physical Science.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalyst Definition Physical Science A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. Hence a catalyst can be recovered chemically unchanged at the end of the reaction it has. Industrial applications of catalysts include. A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; They only. Catalyst Definition Physical Science.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Catalyst Definition Physical Science A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts do not alter the equilibrium position of a reaction; A catalyst is a chemical substance that affects the rate of a chemical reaction by altering. Catalyst Definition Physical Science.
From dxoyvcvpn.blob.core.windows.net
Catalyst Definition In Biology at Esteban Gurley blog Catalyst Definition Physical Science Industrial applications of catalysts include. Catalysts do not alter the equilibrium position of a reaction; Hence a catalyst can be recovered chemically unchanged at the end of the reaction it has. They only help achieve equilibrium faster. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. A catalyst is. Catalyst Definition Physical Science.
From www.youtube.com
How does a CATALYST work ? YouTube Catalyst Definition Physical Science Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. Hence a catalyst can be recovered chemically. Catalyst Definition Physical Science.
From www.worksheetsplanet.com
What is a Catalyst Definition of Catalyst Catalyst Definition Physical Science A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst is some material that speeds up chemical reactions. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Industrial applications of catalysts include. They only help achieve. Catalyst Definition Physical Science.
From examples.yourdictionary.com
Examples of Catalysts YourDictionary Catalyst Definition Physical Science A catalyst is a substance that increases the rate of a chemical reaction without being consumed or permanently altered by the reaction itself. Catalysts do not alter the equilibrium position of a reaction; Industrial applications of catalysts include. A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. Catalyst, in chemistry,. Catalyst Definition Physical Science.
From www.thoughtco.com
Catalyst Definition as Used in Chemistry Catalyst Definition Physical Science A catalyst is some material that speeds up chemical reactions. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Catalysts work by providing an. A catalyst is a substance that increases the rate of a chemical reaction without being consumed or permanently altered by the reaction itself. They only. Catalyst Definition Physical Science.
From www.youtube.com
Definition of Catalyst Chemical Chemistry Class 12 YouTube Catalyst Definition Physical Science Catalysts do not alter the equilibrium position of a reaction; A catalyst is a substance that increases the rate of a chemical reaction without being consumed or permanently altered by the reaction itself. A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; They only help achieve equilibrium faster. With a helping. Catalyst Definition Physical Science.
From www.youtube.com
Heterogeneous catalyst & catalysis 12th Std Chemistry Science Catalyst Definition Physical Science Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts work by providing an. A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. They only help achieve equilibrium faster. A catalyst is a substance that speeds up a chemical reaction, but is not. Catalyst Definition Physical Science.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of Catalyst Definition Physical Science A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. Industrial applications of catalysts include. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. With a helping hand from a catalyst, molecules that might take. Catalyst Definition Physical Science.
From medicaltaste.weebly.com
Periodic table catalyst definition chemistry medicaltaste Catalyst Definition Physical Science A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst is some material that speeds up chemical reactions. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Catalysts work by providing an.. Catalyst Definition Physical Science.
From www.thoughtco.com
Catalysis Definition in Chemistry Catalyst Definition Physical Science Catalysts do not alter the equilibrium position of a reaction; A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. They only help achieve equilibrium faster. A catalyst is some material that speeds up chemical reactions. A catalyst is a substance that increases the rate. Catalyst Definition Physical Science.
From sheetalschemblog.blogspot.com
Sheetal's Chemistry Blog 6.2.5,6.2.6 and 6.2.7 Catalyst Definition Physical Science A catalyst is a substance that increases the rate of a chemical reaction without being consumed or permanently altered by the reaction itself. They only help achieve equilibrium faster. A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; A catalyst is a substance that increases the rate of a chemical reaction. Catalyst Definition Physical Science.
From ppt-online.org
Rates of reaction презентация онлайн Catalyst Definition Physical Science A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; Catalysts do not alter the equilibrium position of a reaction; They only help achieve equilibrium faster. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. With. Catalyst Definition Physical Science.
From scitechdaily.com
Science Made Simple What Are Catalysts? Catalyst Definition Physical Science With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Hence a catalyst can be recovered chemically unchanged at the end of the reaction it has. A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. A catalyst is some material. Catalyst Definition Physical Science.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Catalyst Definition Physical Science A catalyst is a substance that increases the rate of a chemical reaction without being consumed or permanently altered by the reaction itself. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Industrial applications of catalysts include. A catalyst is a substance that increases. Catalyst Definition Physical Science.
From www.youtube.com
Catalytic Converter Working Principle 2 way and 3 way, Function of Catalyst Definition Physical Science A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. They only help achieve equilibrium faster. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that increases the rate of a chemical reaction without being consumed or permanently altered. Catalyst Definition Physical Science.
From brainly.in
define catalyst with example ?? Brainly.in Catalyst Definition Physical Science A catalyst is some material that speeds up chemical reactions. A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. Catalysts do not alter the equilibrium position of a reaction; With a helping. Catalyst Definition Physical Science.