What Metals Can Replace Tin . Tin bonds readily to iron and is used for coating lead, zinc, and steel to prevent corrosion. The metal that does the replacing is always more reactive than the replaced metal. Both aluminum and tin are relatively reactive metals. Tin plated metal from a can. It is used to determine the products of single displacement. The activity series of metals is an empirical tool used to predict products in displacement reactions and reactivity of metals with water and acids in replacement. In general, the more reactive a metal is: Aluminum reacts with oxygen in the air to form aluminum oxide, which provides its. 9 rows the reactivity series of metals is a chart listing metals in order of decreasing reactivity. The reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order. The reactivity series is a series of metals, in order of reactivity from highest to lowest.
from question.pandai.org
The reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order. The metal that does the replacing is always more reactive than the replaced metal. The reactivity series is a series of metals, in order of reactivity from highest to lowest. Aluminum reacts with oxygen in the air to form aluminum oxide, which provides its. 9 rows the reactivity series of metals is a chart listing metals in order of decreasing reactivity. It is used to determine the products of single displacement. The activity series of metals is an empirical tool used to predict products in displacement reactions and reactivity of metals with water and acids in replacement. Tin plated metal from a can. Tin bonds readily to iron and is used for coating lead, zinc, and steel to prevent corrosion. In general, the more reactive a metal is:
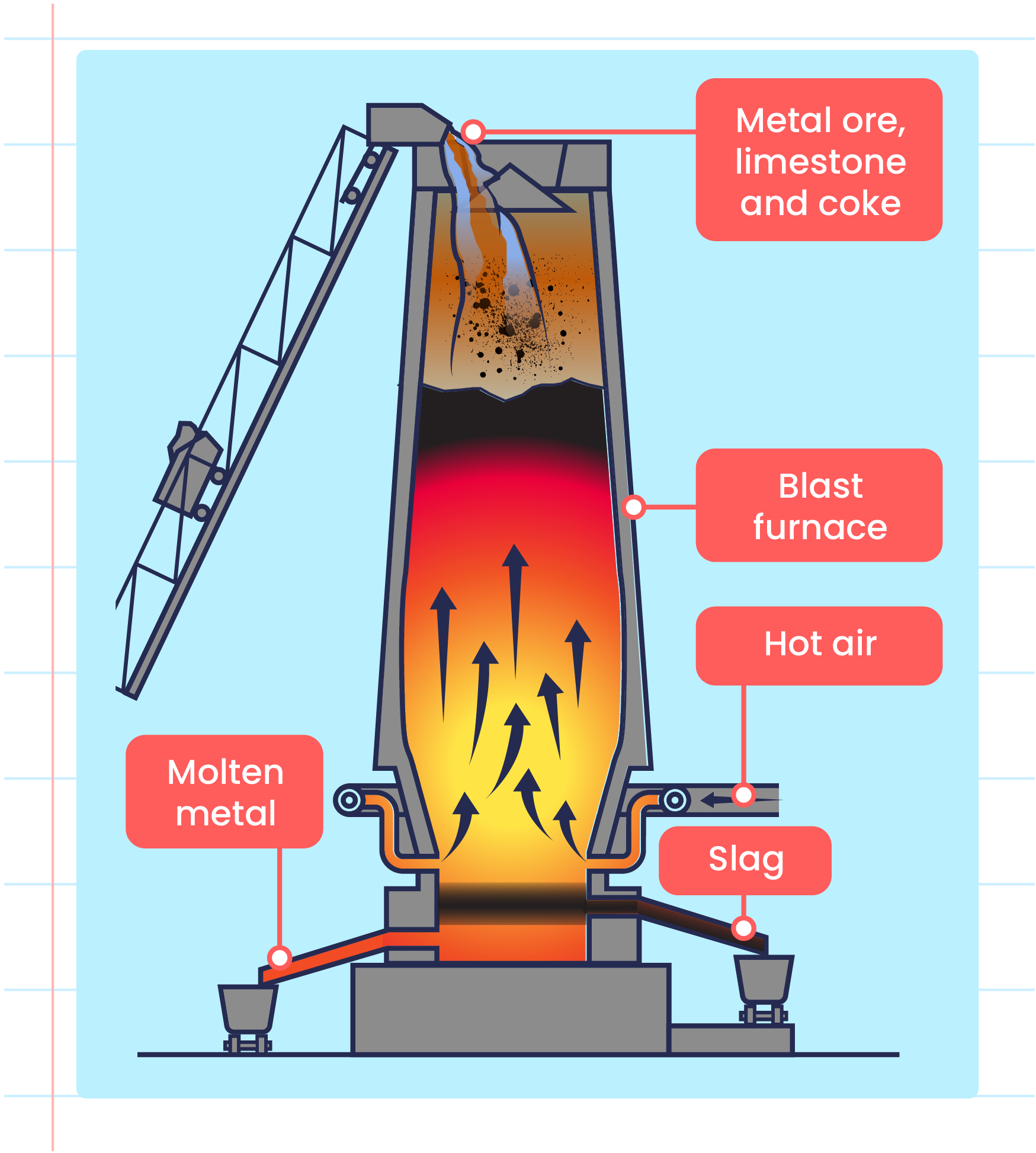
Extraction of Metals from its Ore
What Metals Can Replace Tin It is used to determine the products of single displacement. The metal that does the replacing is always more reactive than the replaced metal. Tin plated metal from a can. Aluminum reacts with oxygen in the air to form aluminum oxide, which provides its. The reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order. The reactivity series is a series of metals, in order of reactivity from highest to lowest. The activity series of metals is an empirical tool used to predict products in displacement reactions and reactivity of metals with water and acids in replacement. 9 rows the reactivity series of metals is a chart listing metals in order of decreasing reactivity. Tin bonds readily to iron and is used for coating lead, zinc, and steel to prevent corrosion. In general, the more reactive a metal is: It is used to determine the products of single displacement. Both aluminum and tin are relatively reactive metals.
From www.argos-st.com
Tin Plating Tinning Treatments for Metals Argos ST What Metals Can Replace Tin The activity series of metals is an empirical tool used to predict products in displacement reactions and reactivity of metals with water and acids in replacement. It is used to determine the products of single displacement. The reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order. In general, the. What Metals Can Replace Tin.
From stlcityrecycles.com
Metal Food and Beverage Cans Part I Saint Louis City Recycles What Metals Can Replace Tin Tin bonds readily to iron and is used for coating lead, zinc, and steel to prevent corrosion. The activity series of metals is an empirical tool used to predict products in displacement reactions and reactivity of metals with water and acids in replacement. The reactivity series is a series of metals, in order of reactivity from highest to lowest. It. What Metals Can Replace Tin.
From www.belmontmetals.com
Applications and Uses for Tin Sheet Belmont Metals What Metals Can Replace Tin Both aluminum and tin are relatively reactive metals. 9 rows the reactivity series of metals is a chart listing metals in order of decreasing reactivity. Tin bonds readily to iron and is used for coating lead, zinc, and steel to prevent corrosion. The reactivity series of metals, also known as the activity series, refers to the arrangement of metals in. What Metals Can Replace Tin.
From spmscience.blog.onlinetuition.com.my
5.4.3 Extraction of Metals from their Ores Using Coke SPM Science What Metals Can Replace Tin Aluminum reacts with oxygen in the air to form aluminum oxide, which provides its. The activity series of metals is an empirical tool used to predict products in displacement reactions and reactivity of metals with water and acids in replacement. It is used to determine the products of single displacement. The metal that does the replacing is always more reactive. What Metals Can Replace Tin.
From www.greelane.com
Metaller Nasıl ve Neden Aşınır? What Metals Can Replace Tin It is used to determine the products of single displacement. Tin plated metal from a can. The metal that does the replacing is always more reactive than the replaced metal. In general, the more reactive a metal is: Both aluminum and tin are relatively reactive metals. 9 rows the reactivity series of metals is a chart listing metals in order. What Metals Can Replace Tin.
From exofshwix.blob.core.windows.net
Activity Table Of Metals at Lou Huey blog What Metals Can Replace Tin Tin plated metal from a can. 9 rows the reactivity series of metals is a chart listing metals in order of decreasing reactivity. It is used to determine the products of single displacement. The reactivity series is a series of metals, in order of reactivity from highest to lowest. The activity series of metals is an empirical tool used to. What Metals Can Replace Tin.
From fphoto.photoshelter.com
science element carbon group 14 Fundamental Photographs The Art of What Metals Can Replace Tin It is used to determine the products of single displacement. The activity series of metals is an empirical tool used to predict products in displacement reactions and reactivity of metals with water and acids in replacement. The reactivity series is a series of metals, in order of reactivity from highest to lowest. Aluminum reacts with oxygen in the air to. What Metals Can Replace Tin.
From www.electricallicenserenewal.com
358.14 Dissimilar Metals. What Metals Can Replace Tin Aluminum reacts with oxygen in the air to form aluminum oxide, which provides its. 9 rows the reactivity series of metals is a chart listing metals in order of decreasing reactivity. The activity series of metals is an empirical tool used to predict products in displacement reactions and reactivity of metals with water and acids in replacement. Tin bonds readily. What Metals Can Replace Tin.
From www.youtube.com
Stepbystep how to DIY Install Metal Skirting on a Mobile home or What Metals Can Replace Tin The reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order. It is used to determine the products of single displacement. In general, the more reactive a metal is: Aluminum reacts with oxygen in the air to form aluminum oxide, which provides its. The activity series of metals is an. What Metals Can Replace Tin.
From www.indium.com
Tin Metals and Alloys Products made by Indium Corporation What Metals Can Replace Tin Aluminum reacts with oxygen in the air to form aluminum oxide, which provides its. It is used to determine the products of single displacement. Tin plated metal from a can. Both aluminum and tin are relatively reactive metals. The reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order. The. What Metals Can Replace Tin.
From testbook.com
[Solved] Select the set of least reactive metals. What Metals Can Replace Tin Aluminum reacts with oxygen in the air to form aluminum oxide, which provides its. The activity series of metals is an empirical tool used to predict products in displacement reactions and reactivity of metals with water and acids in replacement. 9 rows the reactivity series of metals is a chart listing metals in order of decreasing reactivity. The reactivity series. What Metals Can Replace Tin.
From mammothmemory.net
The reaction between tin and oxygen What Metals Can Replace Tin The reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order. The metal that does the replacing is always more reactive than the replaced metal. In general, the more reactive a metal is: 9 rows the reactivity series of metals is a chart listing metals in order of decreasing reactivity.. What Metals Can Replace Tin.
From lectromec.com
What are the Potential Issues with Tin Plated Conductors? Lectromec What Metals Can Replace Tin Tin bonds readily to iron and is used for coating lead, zinc, and steel to prevent corrosion. The reactivity series is a series of metals, in order of reactivity from highest to lowest. Aluminum reacts with oxygen in the air to form aluminum oxide, which provides its. The metal that does the replacing is always more reactive than the replaced. What Metals Can Replace Tin.
From study.com
What is the Element Tin Used For? Lesson For Kids Lesson What Metals Can Replace Tin In general, the more reactive a metal is: Aluminum reacts with oxygen in the air to form aluminum oxide, which provides its. The metal that does the replacing is always more reactive than the replaced metal. Both aluminum and tin are relatively reactive metals. Tin plated metal from a can. Tin bonds readily to iron and is used for coating. What Metals Can Replace Tin.
From www.slideshare.net
Metal Product Display On Tin What Metals Can Replace Tin 9 rows the reactivity series of metals is a chart listing metals in order of decreasing reactivity. It is used to determine the products of single displacement. The metal that does the replacing is always more reactive than the replaced metal. The activity series of metals is an empirical tool used to predict products in displacement reactions and reactivity of. What Metals Can Replace Tin.
From www.andreabonelli.com
Guide to metal colors Andrea Bonelli What Metals Can Replace Tin Aluminum reacts with oxygen in the air to form aluminum oxide, which provides its. The reactivity series is a series of metals, in order of reactivity from highest to lowest. 9 rows the reactivity series of metals is a chart listing metals in order of decreasing reactivity. Tin plated metal from a can. The activity series of metals is an. What Metals Can Replace Tin.
From www.doubtnut.com
How many of the following metals can replace Sn(Tin) from its compound What Metals Can Replace Tin The metal that does the replacing is always more reactive than the replaced metal. The reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order. The activity series of metals is an empirical tool used to predict products in displacement reactions and reactivity of metals with water and acids in. What Metals Can Replace Tin.
From slideplayer.com
PAP Chemistry Bell Ringer Schedule Thursday 2/9/12 Bell Ringer ppt What Metals Can Replace Tin The metal that does the replacing is always more reactive than the replaced metal. The activity series of metals is an empirical tool used to predict products in displacement reactions and reactivity of metals with water and acids in replacement. Tin bonds readily to iron and is used for coating lead, zinc, and steel to prevent corrosion. Aluminum reacts with. What Metals Can Replace Tin.
From www.teachoo.com
Reaction of Metals and Nonmetals with Oxygen Concepts What Metals Can Replace Tin In general, the more reactive a metal is: The reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order. It is used to determine the products of single displacement. 9 rows the reactivity series of metals is a chart listing metals in order of decreasing reactivity. The activity series of. What Metals Can Replace Tin.
From sites.google.com
Tin Metals What Metals Can Replace Tin 9 rows the reactivity series of metals is a chart listing metals in order of decreasing reactivity. Aluminum reacts with oxygen in the air to form aluminum oxide, which provides its. It is used to determine the products of single displacement. The activity series of metals is an empirical tool used to predict products in displacement reactions and reactivity of. What Metals Can Replace Tin.
From images-of-elements.com
Chemical Elements Tin What Metals Can Replace Tin It is used to determine the products of single displacement. Tin bonds readily to iron and is used for coating lead, zinc, and steel to prevent corrosion. The metal that does the replacing is always more reactive than the replaced metal. The reactivity series is a series of metals, in order of reactivity from highest to lowest. Both aluminum and. What Metals Can Replace Tin.
From www.tinboxmate.com
metal tin box from Bowang tin box factory, accept tin box customized What Metals Can Replace Tin It is used to determine the products of single displacement. The reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order. 9 rows the reactivity series of metals is a chart listing metals in order of decreasing reactivity. The reactivity series is a series of metals, in order of reactivity. What Metals Can Replace Tin.
From www.zogirls.com
金属的反应性 AQA GCSE化学综合科学修订笔记2018 拯救我的考试 What Metals Can Replace Tin Aluminum reacts with oxygen in the air to form aluminum oxide, which provides its. It is used to determine the products of single displacement. Both aluminum and tin are relatively reactive metals. The metal that does the replacing is always more reactive than the replaced metal. 9 rows the reactivity series of metals is a chart listing metals in order. What Metals Can Replace Tin.
From exofrimre.blob.core.windows.net
How To Install Tin On Exterior Walls at Adolfo Peralta blog What Metals Can Replace Tin 9 rows the reactivity series of metals is a chart listing metals in order of decreasing reactivity. The activity series of metals is an empirical tool used to predict products in displacement reactions and reactivity of metals with water and acids in replacement. The reactivity series is a series of metals, in order of reactivity from highest to lowest. The. What Metals Can Replace Tin.
From aerospacemetalsllc.com
What is Tin Plating? Aerospace Metals What Metals Can Replace Tin The reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order. The activity series of metals is an empirical tool used to predict products in displacement reactions and reactivity of metals with water and acids in replacement. 9 rows the reactivity series of metals is a chart listing metals in. What Metals Can Replace Tin.
From www.slideserve.com
PPT CHAPTER 4 MATERIALS METALS AND NON METALS PowerPoint What Metals Can Replace Tin In general, the more reactive a metal is: The activity series of metals is an empirical tool used to predict products in displacement reactions and reactivity of metals with water and acids in replacement. Aluminum reacts with oxygen in the air to form aluminum oxide, which provides its. The reactivity series is a series of metals, in order of reactivity. What Metals Can Replace Tin.
From question.pandai.org
Extraction of Metals from its Ore What Metals Can Replace Tin It is used to determine the products of single displacement. The metal that does the replacing is always more reactive than the replaced metal. Both aluminum and tin are relatively reactive metals. The reactivity series is a series of metals, in order of reactivity from highest to lowest. Aluminum reacts with oxygen in the air to form aluminum oxide, which. What Metals Can Replace Tin.
From www.pinterest.com
Materials Types of metal, Metal, Hello english app What Metals Can Replace Tin Tin bonds readily to iron and is used for coating lead, zinc, and steel to prevent corrosion. It is used to determine the products of single displacement. The reactivity series is a series of metals, in order of reactivity from highest to lowest. Both aluminum and tin are relatively reactive metals. The activity series of metals is an empirical tool. What Metals Can Replace Tin.
From metal-forex-trader.blogspot.com
Commodity Trading News And Technical Analysis Reports. Edward Meir What Metals Can Replace Tin The reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order. Tin plated metal from a can. Aluminum reacts with oxygen in the air to form aluminum oxide, which provides its. It is used to determine the products of single displacement. Both aluminum and tin are relatively reactive metals. The. What Metals Can Replace Tin.
From www.thoughtco.com
Transition Metals — Properties of the Element Group What Metals Can Replace Tin The reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order. The metal that does the replacing is always more reactive than the replaced metal. 9 rows the reactivity series of metals is a chart listing metals in order of decreasing reactivity. Tin bonds readily to iron and is used. What Metals Can Replace Tin.
From sciencenotes.org
Activity Series of Metals (Reactivity Series) What Metals Can Replace Tin It is used to determine the products of single displacement. Both aluminum and tin are relatively reactive metals. The metal that does the replacing is always more reactive than the replaced metal. The reactivity series is a series of metals, in order of reactivity from highest to lowest. The activity series of metals is an empirical tool used to predict. What Metals Can Replace Tin.
From sizermetal.com
Tin Sizer Metal Singapore What Metals Can Replace Tin Aluminum reacts with oxygen in the air to form aluminum oxide, which provides its. The reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order. Both aluminum and tin are relatively reactive metals. Tin plated metal from a can. 9 rows the reactivity series of metals is a chart listing. What Metals Can Replace Tin.
From www.dreamstime.com
Metal Cans And Tins Prepared For Recycling Stock Image Image 37352267 What Metals Can Replace Tin The reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order. The reactivity series is a series of metals, in order of reactivity from highest to lowest. Tin bonds readily to iron and is used for coating lead, zinc, and steel to prevent corrosion. 9 rows the reactivity series of. What Metals Can Replace Tin.
From www.cadillacforums.com
Visualizing 25 Years of Lithium Production, by Country and other What Metals Can Replace Tin It is used to determine the products of single displacement. The reactivity series is a series of metals, in order of reactivity from highest to lowest. The metal that does the replacing is always more reactive than the replaced metal. 9 rows the reactivity series of metals is a chart listing metals in order of decreasing reactivity. Tin bonds readily. What Metals Can Replace Tin.
From www.roboze.com
Metal replacement why replace metals with polymers and composites? What Metals Can Replace Tin Both aluminum and tin are relatively reactive metals. In general, the more reactive a metal is: The reactivity series is a series of metals, in order of reactivity from highest to lowest. Tin plated metal from a can. The reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order. 9. What Metals Can Replace Tin.