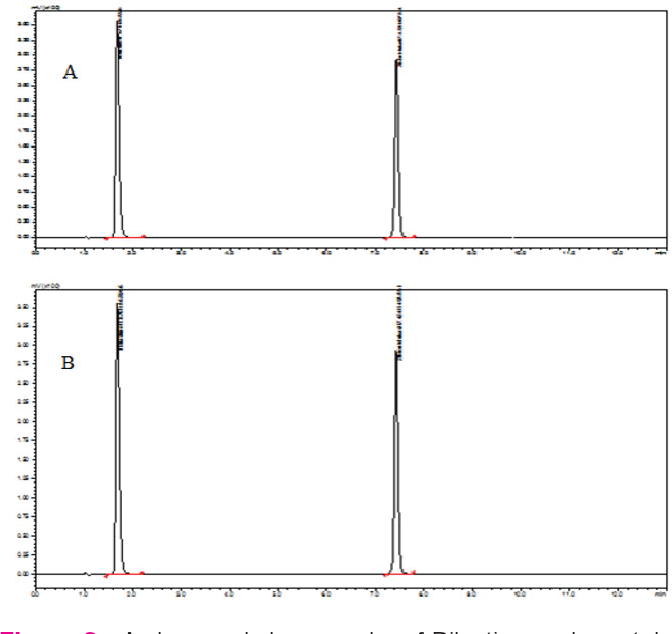
Hplc Method Development And Validation Of Primaquine . In this work, a new isocratic reverse phase chromatographic method was developed using uplc for primaquine phosphate bulk drug. The newly developed uplc method for related substance and assay determination of primaquine phosphate was found to be capable. The chromatographic separation of primaquine and impurities was achieved on a waters acquity beh c18, 50 x 2.1mm, 1.7. Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile.
from www.semanticscholar.org
Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. The chromatographic separation of primaquine and impurities was achieved on a waters acquity beh c18, 50 x 2.1mm, 1.7. In this work, a new isocratic reverse phase chromatographic method was developed using uplc for primaquine phosphate bulk drug. The newly developed uplc method for related substance and assay determination of primaquine phosphate was found to be capable.
Figure 1 from A New RPHPLC Assay Method Development and Validation for
Hplc Method Development And Validation Of Primaquine The newly developed uplc method for related substance and assay determination of primaquine phosphate was found to be capable. Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. The chromatographic separation of primaquine and impurities was achieved on a waters acquity beh c18, 50 x 2.1mm, 1.7. In this work, a new isocratic reverse phase chromatographic method was developed using uplc for primaquine phosphate bulk drug. The newly developed uplc method for related substance and assay determination of primaquine phosphate was found to be capable.
From www.semanticscholar.org
Figure 1 from A New RPHPLC Assay Method Development and Validation for Hplc Method Development And Validation Of Primaquine Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. The newly developed uplc method for related substance and assay determination of primaquine phosphate was found to be capable. The chromatographic separation of primaquine and impurities was achieved on a waters acquity beh c18, 50 x 2.1mm, 1.7. In this work, a. Hplc Method Development And Validation Of Primaquine.
From www.youtube.com
HPLC Method Validation HPLC System Suitability Analytical Method Hplc Method Development And Validation Of Primaquine In this work, a new isocratic reverse phase chromatographic method was developed using uplc for primaquine phosphate bulk drug. The chromatographic separation of primaquine and impurities was achieved on a waters acquity beh c18, 50 x 2.1mm, 1.7. Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. The newly developed uplc. Hplc Method Development And Validation Of Primaquine.
From www.researchgate.net
Steps involved in HPLC method development Download Scientific Diagram Hplc Method Development And Validation Of Primaquine The chromatographic separation of primaquine and impurities was achieved on a waters acquity beh c18, 50 x 2.1mm, 1.7. Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. In this work, a new isocratic reverse phase chromatographic method was developed using uplc for primaquine phosphate bulk drug. The newly developed uplc. Hplc Method Development And Validation Of Primaquine.
From ymc.eu
HPLC Method Development and Validation Kits Hplc Method Development And Validation Of Primaquine The chromatographic separation of primaquine and impurities was achieved on a waters acquity beh c18, 50 x 2.1mm, 1.7. Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. The newly developed uplc method for related substance and assay determination of primaquine phosphate was found to be capable. In this work, a. Hplc Method Development And Validation Of Primaquine.
From studylib.net
Development, validation, and comparison of an HPLC method to Hplc Method Development And Validation Of Primaquine In this work, a new isocratic reverse phase chromatographic method was developed using uplc for primaquine phosphate bulk drug. The newly developed uplc method for related substance and assay determination of primaquine phosphate was found to be capable. The chromatographic separation of primaquine and impurities was achieved on a waters acquity beh c18, 50 x 2.1mm, 1.7. Thus, hplc and. Hplc Method Development And Validation Of Primaquine.
From www.tpsearchtool.com
Hplc Method Development And Validation For Pharmaceutical Analysis Images Hplc Method Development And Validation Of Primaquine The chromatographic separation of primaquine and impurities was achieved on a waters acquity beh c18, 50 x 2.1mm, 1.7. Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. The newly developed uplc method for related substance and assay determination of primaquine phosphate was found to be capable. In this work, a. Hplc Method Development And Validation Of Primaquine.
From www.researchgate.net
(PDF) RPHPLC method development and validation for estimation of Hplc Method Development And Validation Of Primaquine Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. The chromatographic separation of primaquine and impurities was achieved on a waters acquity beh c18, 50 x 2.1mm, 1.7. In this work, a new isocratic reverse phase chromatographic method was developed using uplc for primaquine phosphate bulk drug. The newly developed uplc. Hplc Method Development And Validation Of Primaquine.
From ciinformatics.co.uk
HPLC Method Development Software Fusion LC Method Development Hplc Method Development And Validation Of Primaquine The chromatographic separation of primaquine and impurities was achieved on a waters acquity beh c18, 50 x 2.1mm, 1.7. The newly developed uplc method for related substance and assay determination of primaquine phosphate was found to be capable. Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. In this work, a. Hplc Method Development And Validation Of Primaquine.
From www.morebooks.de
HPLC Method Development and Validation in Pharmaceutical Analysis, 978 Hplc Method Development And Validation Of Primaquine Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. In this work, a new isocratic reverse phase chromatographic method was developed using uplc for primaquine phosphate bulk drug. The chromatographic separation of primaquine and impurities was achieved on a waters acquity beh c18, 50 x 2.1mm, 1.7. The newly developed uplc. Hplc Method Development And Validation Of Primaquine.
From www.slideshare.net
analytical method validation and validation of hplc Hplc Method Development And Validation Of Primaquine The newly developed uplc method for related substance and assay determination of primaquine phosphate was found to be capable. Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. The chromatographic separation of primaquine and impurities was achieved on a waters acquity beh c18, 50 x 2.1mm, 1.7. In this work, a. Hplc Method Development And Validation Of Primaquine.
From www.slideshare.net
Stability indicating rp hplc method development and validation for si… Hplc Method Development And Validation Of Primaquine The newly developed uplc method for related substance and assay determination of primaquine phosphate was found to be capable. The chromatographic separation of primaquine and impurities was achieved on a waters acquity beh c18, 50 x 2.1mm, 1.7. Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. In this work, a. Hplc Method Development And Validation Of Primaquine.
From www.noor-publishing.com
Development and Validation of Stability Indicating RPHPLC Method / 978 Hplc Method Development And Validation Of Primaquine In this work, a new isocratic reverse phase chromatographic method was developed using uplc for primaquine phosphate bulk drug. The chromatographic separation of primaquine and impurities was achieved on a waters acquity beh c18, 50 x 2.1mm, 1.7. Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. The newly developed uplc. Hplc Method Development And Validation Of Primaquine.
From www.semanticscholar.org
[PDF] Development and Validation of RPHPLC Method An Overview Hplc Method Development And Validation Of Primaquine In this work, a new isocratic reverse phase chromatographic method was developed using uplc for primaquine phosphate bulk drug. The chromatographic separation of primaquine and impurities was achieved on a waters acquity beh c18, 50 x 2.1mm, 1.7. Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. The newly developed uplc. Hplc Method Development And Validation Of Primaquine.
From www.scielo.br
SciELO Brasil A newly validated highperformance liquid Hplc Method Development And Validation Of Primaquine Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. The chromatographic separation of primaquine and impurities was achieved on a waters acquity beh c18, 50 x 2.1mm, 1.7. The newly developed uplc method for related substance and assay determination of primaquine phosphate was found to be capable. In this work, a. Hplc Method Development And Validation Of Primaquine.
From www.researchgate.net
(PDF) A Review HPLC Method Development and Validation Hplc Method Development And Validation Of Primaquine Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. In this work, a new isocratic reverse phase chromatographic method was developed using uplc for primaquine phosphate bulk drug. The newly developed uplc method for related substance and assay determination of primaquine phosphate was found to be capable. The chromatographic separation of. Hplc Method Development And Validation Of Primaquine.
From dheerajpratapsingh0.blogspot.com
Steps for HPLC Method Development Hplc Method Development And Validation Of Primaquine Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. The newly developed uplc method for related substance and assay determination of primaquine phosphate was found to be capable. The chromatographic separation of primaquine and impurities was achieved on a waters acquity beh c18, 50 x 2.1mm, 1.7. In this work, a. Hplc Method Development And Validation Of Primaquine.
From www.slideshare.net
analytical method validation and validation of hplc Hplc Method Development And Validation Of Primaquine The newly developed uplc method for related substance and assay determination of primaquine phosphate was found to be capable. Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. In this work, a new isocratic reverse phase chromatographic method was developed using uplc for primaquine phosphate bulk drug. The chromatographic separation of. Hplc Method Development And Validation Of Primaquine.
From www.youtube.com
How to do HPLC method validation YouTube Hplc Method Development And Validation Of Primaquine Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. In this work, a new isocratic reverse phase chromatographic method was developed using uplc for primaquine phosphate bulk drug. The newly developed uplc method for related substance and assay determination of primaquine phosphate was found to be capable. The chromatographic separation of. Hplc Method Development And Validation Of Primaquine.
From www.lap-publishing.com
Practical HPLC and LCMS Method Development and Validation / 9783659 Hplc Method Development And Validation Of Primaquine In this work, a new isocratic reverse phase chromatographic method was developed using uplc for primaquine phosphate bulk drug. The newly developed uplc method for related substance and assay determination of primaquine phosphate was found to be capable. The chromatographic separation of primaquine and impurities was achieved on a waters acquity beh c18, 50 x 2.1mm, 1.7. Thus, hplc and. Hplc Method Development And Validation Of Primaquine.
From www.slideshare.net
analytical method validation and validation of hplc Hplc Method Development And Validation Of Primaquine The newly developed uplc method for related substance and assay determination of primaquine phosphate was found to be capable. Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. The chromatographic separation of primaquine and impurities was achieved on a waters acquity beh c18, 50 x 2.1mm, 1.7. In this work, a. Hplc Method Development And Validation Of Primaquine.
From www.morebooks.de
HPTLC Method Development and Validation, 9783844327359, 3844327355 Hplc Method Development And Validation Of Primaquine Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. The newly developed uplc method for related substance and assay determination of primaquine phosphate was found to be capable. In this work, a new isocratic reverse phase chromatographic method was developed using uplc for primaquine phosphate bulk drug. The chromatographic separation of. Hplc Method Development And Validation Of Primaquine.
From informacionpublica.svet.gob.gt
How To Do HPLC Method Validation Hplc Method Development And Validation Of Primaquine Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. The chromatographic separation of primaquine and impurities was achieved on a waters acquity beh c18, 50 x 2.1mm, 1.7. The newly developed uplc method for related substance and assay determination of primaquine phosphate was found to be capable. In this work, a. Hplc Method Development And Validation Of Primaquine.
From www.slideshare.net
DEVELOPMENT AND VALIDATION OF STABILITY INDICATING RPHPLC METHOD F… Hplc Method Development And Validation Of Primaquine The newly developed uplc method for related substance and assay determination of primaquine phosphate was found to be capable. In this work, a new isocratic reverse phase chromatographic method was developed using uplc for primaquine phosphate bulk drug. The chromatographic separation of primaquine and impurities was achieved on a waters acquity beh c18, 50 x 2.1mm, 1.7. Thus, hplc and. Hplc Method Development And Validation Of Primaquine.
From www.slideshare.net
analytical method validation and validation of hplc Hplc Method Development And Validation Of Primaquine In this work, a new isocratic reverse phase chromatographic method was developed using uplc for primaquine phosphate bulk drug. The chromatographic separation of primaquine and impurities was achieved on a waters acquity beh c18, 50 x 2.1mm, 1.7. Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. The newly developed uplc. Hplc Method Development And Validation Of Primaquine.
From pubs.acs.org
Rapid Analytical Method Development and Validation of RPHPLC Method Hplc Method Development And Validation Of Primaquine The chromatographic separation of primaquine and impurities was achieved on a waters acquity beh c18, 50 x 2.1mm, 1.7. In this work, a new isocratic reverse phase chromatographic method was developed using uplc for primaquine phosphate bulk drug. Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. The newly developed uplc. Hplc Method Development And Validation Of Primaquine.
From issuu.com
Significance of HPLC Method Development, Validation, and Sample Hplc Method Development And Validation Of Primaquine The newly developed uplc method for related substance and assay determination of primaquine phosphate was found to be capable. In this work, a new isocratic reverse phase chromatographic method was developed using uplc for primaquine phosphate bulk drug. Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. The chromatographic separation of. Hplc Method Development And Validation Of Primaquine.
From www.researchgate.net
(PDF) ANALYTICAL METHOD DEVELOPMENT AND VALIDATION FOR THE SIMULTANEOUS Hplc Method Development And Validation Of Primaquine The newly developed uplc method for related substance and assay determination of primaquine phosphate was found to be capable. Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. In this work, a new isocratic reverse phase chromatographic method was developed using uplc for primaquine phosphate bulk drug. The chromatographic separation of. Hplc Method Development And Validation Of Primaquine.
From mungfali.com
Table 1 From Optimization And Validation Of Rphplc Method CA3 Hplc Method Development And Validation Of Primaquine In this work, a new isocratic reverse phase chromatographic method was developed using uplc for primaquine phosphate bulk drug. The chromatographic separation of primaquine and impurities was achieved on a waters acquity beh c18, 50 x 2.1mm, 1.7. The newly developed uplc method for related substance and assay determination of primaquine phosphate was found to be capable. Thus, hplc and. Hplc Method Development And Validation Of Primaquine.
From dokumen.tips
(PDF) A REVIEW HPLC METHOD DEVELOPMENT AND VALIDATION DOKUMEN.TIPS Hplc Method Development And Validation Of Primaquine Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. In this work, a new isocratic reverse phase chromatographic method was developed using uplc for primaquine phosphate bulk drug. The newly developed uplc method for related substance and assay determination of primaquine phosphate was found to be capable. The chromatographic separation of. Hplc Method Development And Validation Of Primaquine.
From www.researchgate.net
HPLC method development strategy. Download Scientific Diagram Hplc Method Development And Validation Of Primaquine The chromatographic separation of primaquine and impurities was achieved on a waters acquity beh c18, 50 x 2.1mm, 1.7. In this work, a new isocratic reverse phase chromatographic method was developed using uplc for primaquine phosphate bulk drug. Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. The newly developed uplc. Hplc Method Development And Validation Of Primaquine.
From www.semanticscholar.org
Figure 2 from Analysis of primaquine and its metabolite Hplc Method Development And Validation Of Primaquine The newly developed uplc method for related substance and assay determination of primaquine phosphate was found to be capable. In this work, a new isocratic reverse phase chromatographic method was developed using uplc for primaquine phosphate bulk drug. The chromatographic separation of primaquine and impurities was achieved on a waters acquity beh c18, 50 x 2.1mm, 1.7. Thus, hplc and. Hplc Method Development And Validation Of Primaquine.
From www.semanticscholar.org
Figure 1 from Development and Validation of RPHPLC Method for Hplc Method Development And Validation Of Primaquine Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. In this work, a new isocratic reverse phase chromatographic method was developed using uplc for primaquine phosphate bulk drug. The newly developed uplc method for related substance and assay determination of primaquine phosphate was found to be capable. The chromatographic separation of. Hplc Method Development And Validation Of Primaquine.
From www.researchgate.net
Structures of Primaquine and Quinocide Primaquine Download Scientific Hplc Method Development And Validation Of Primaquine The chromatographic separation of primaquine and impurities was achieved on a waters acquity beh c18, 50 x 2.1mm, 1.7. In this work, a new isocratic reverse phase chromatographic method was developed using uplc for primaquine phosphate bulk drug. The newly developed uplc method for related substance and assay determination of primaquine phosphate was found to be capable. Thus, hplc and. Hplc Method Development And Validation Of Primaquine.
From www.semanticscholar.org
Bioanalytical Method Development and Validation by HPLC A Review Hplc Method Development And Validation Of Primaquine The chromatographic separation of primaquine and impurities was achieved on a waters acquity beh c18, 50 x 2.1mm, 1.7. Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. The newly developed uplc method for related substance and assay determination of primaquine phosphate was found to be capable. In this work, a. Hplc Method Development And Validation Of Primaquine.
From www.researchgate.net
(PDF) Development and Validation of RPHPLC Method for the Simultaneous Hplc Method Development And Validation Of Primaquine The chromatographic separation of primaquine and impurities was achieved on a waters acquity beh c18, 50 x 2.1mm, 1.7. In this work, a new isocratic reverse phase chromatographic method was developed using uplc for primaquine phosphate bulk drug. Thus, hplc and uplc methods showed to be robust regarding chloroquine and primaquine contents for changes in acetonitrile. The newly developed uplc. Hplc Method Development And Validation Of Primaquine.