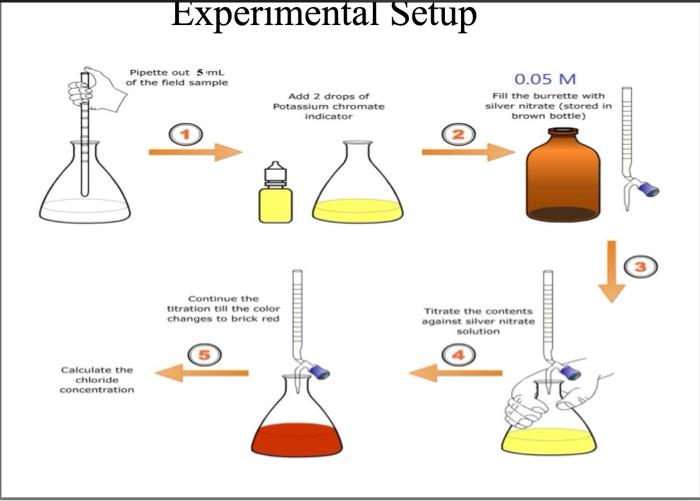
Indicator For Chloride Titration . As the silver nitrate solution is slowly. The determination of chloride by the mohr method. Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently used titrimetric methods of analysis. Titrant is used when the chloride concentration is less than 200 mg/l. In the volhard method chlorides are first precipitated with excess silver nitrate, then excess silver is titrated with potassium (or. However, the end point is not as sharp as when a more concentrated titrant is. Discussion the mohr method uses chromate ion as an indicator in the titration of. The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. This method determines the chloride ion concentration of a solution by titration with silver nitrate.
from www.chegg.com
Discussion the mohr method uses chromate ion as an indicator in the titration of. The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. Titrant is used when the chloride concentration is less than 200 mg/l. The determination of chloride by the mohr method. In the volhard method chlorides are first precipitated with excess silver nitrate, then excess silver is titrated with potassium (or. This method determines the chloride ion concentration of a solution by titration with silver nitrate. However, the end point is not as sharp as when a more concentrated titrant is. Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently used titrimetric methods of analysis. As the silver nitrate solution is slowly.
Solved Experiment 8 Determination of chloride ion in water
Indicator For Chloride Titration However, the end point is not as sharp as when a more concentrated titrant is. In the volhard method chlorides are first precipitated with excess silver nitrate, then excess silver is titrated with potassium (or. Discussion the mohr method uses chromate ion as an indicator in the titration of. The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. Titrant is used when the chloride concentration is less than 200 mg/l. The determination of chloride by the mohr method. This method determines the chloride ion concentration of a solution by titration with silver nitrate. As the silver nitrate solution is slowly. Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently used titrimetric methods of analysis. However, the end point is not as sharp as when a more concentrated titrant is.
From www.lazada.com.ph
Reagent silver nitrate standard solution chloride ion water quality Indicator For Chloride Titration However, the end point is not as sharp as when a more concentrated titrant is. The determination of chloride by the mohr method. The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. In the volhard method chlorides are first precipitated with excess silver nitrate, then excess. Indicator For Chloride Titration.
From www.researchgate.net
(PDF) IndicatorFree Argentometric Titration for DistanceBased Indicator For Chloride Titration This method determines the chloride ion concentration of a solution by titration with silver nitrate. In the volhard method chlorides are first precipitated with excess silver nitrate, then excess silver is titrated with potassium (or. Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently used titrimetric methods of analysis. Discussion the mohr method uses chromate. Indicator For Chloride Titration.
From letitsnowglobe.co.uk
Titration procedure pdf Indicator For Chloride Titration Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently used titrimetric methods of analysis. Titrant is used when the chloride concentration is less than 200 mg/l. The determination of chloride by the mohr method. However, the end point is not as sharp as when a more concentrated titrant is. As the silver nitrate solution is. Indicator For Chloride Titration.
From www.semanticscholar.org
Figure 2 from Mercurimetric Titration of Chloride in Presenceof Sodium Indicator For Chloride Titration The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. Titrant is used when the chloride concentration is less than 200 mg/l. However, the end point is not as sharp as when a more concentrated titrant is. Discussion the mohr method uses chromate ion as an indicator. Indicator For Chloride Titration.
From mavink.com
Titration Labeled Indicator For Chloride Titration The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently used titrimetric methods of analysis. In the volhard method chlorides are first precipitated with excess silver nitrate, then excess silver is titrated with. Indicator For Chloride Titration.
From aquaspex.com.au
Chloride HG Indicator Indicator For Chloride Titration The determination of chloride by the mohr method. In the volhard method chlorides are first precipitated with excess silver nitrate, then excess silver is titrated with potassium (or. Titrant is used when the chloride concentration is less than 200 mg/l. Discussion the mohr method uses chromate ion as an indicator in the titration of. Beside acid/base titrations, the titrimetric determination. Indicator For Chloride Titration.
From www.youtube.com
TITRATION OF CHLORIDE IONS WITH SILVER NITRATE YouTube Indicator For Chloride Titration Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently used titrimetric methods of analysis. The determination of chloride by the mohr method. The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. As the silver nitrate solution is slowly. However, the end. Indicator For Chloride Titration.
From www.scribd.com
Chloride Mohr Silver Titration Indicator For Chloride Titration Discussion the mohr method uses chromate ion as an indicator in the titration of. Titrant is used when the chloride concentration is less than 200 mg/l. However, the end point is not as sharp as when a more concentrated titrant is. As the silver nitrate solution is slowly. This method determines the chloride ion concentration of a solution by titration. Indicator For Chloride Titration.
From www.chegg.com
Solved Experiment 8 Determination of chloride ion in water Indicator For Chloride Titration Discussion the mohr method uses chromate ion as an indicator in the titration of. In the volhard method chlorides are first precipitated with excess silver nitrate, then excess silver is titrated with potassium (or. This method determines the chloride ion concentration of a solution by titration with silver nitrate. Beside acid/base titrations, the titrimetric determination of chloride is one of. Indicator For Chloride Titration.
From achs-prod.acs.org
IndicatorFree Argentometric Titration for DistanceBased Detection of Indicator For Chloride Titration The determination of chloride by the mohr method. However, the end point is not as sharp as when a more concentrated titrant is. Titrant is used when the chloride concentration is less than 200 mg/l. Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently used titrimetric methods of analysis. As the silver nitrate solution is. Indicator For Chloride Titration.
From theedge.com.hk
Chemistry How To Titration The Edge Indicator For Chloride Titration The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. Discussion the mohr method uses chromate ion as an indicator in the titration of. The determination of chloride by the mohr method. As the silver nitrate solution is slowly. Beside acid/base titrations, the titrimetric determination of chloride. Indicator For Chloride Titration.
From www.chegg.com
Solved Determination of Chloride lon in Seawater by Mohr's Indicator For Chloride Titration In the volhard method chlorides are first precipitated with excess silver nitrate, then excess silver is titrated with potassium (or. Titrant is used when the chloride concentration is less than 200 mg/l. As the silver nitrate solution is slowly. However, the end point is not as sharp as when a more concentrated titrant is. The concentration of chloride ions is. Indicator For Chloride Titration.
From www.semanticscholar.org
Figure 1 from Mercurimetric Titration of Chloride in Presenceof Sodium Indicator For Chloride Titration Discussion the mohr method uses chromate ion as an indicator in the titration of. This method determines the chloride ion concentration of a solution by titration with silver nitrate. As the silver nitrate solution is slowly. However, the end point is not as sharp as when a more concentrated titrant is. The concentration of chloride ions is determined by subtracting. Indicator For Chloride Titration.
From www.numerade.com
Potassium chromate is used as an indicator in titrations for the Indicator For Chloride Titration Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently used titrimetric methods of analysis. Titrant is used when the chloride concentration is less than 200 mg/l. The determination of chloride by the mohr method. As the silver nitrate solution is slowly. Discussion the mohr method uses chromate ion as an indicator in the titration of.. Indicator For Chloride Titration.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Indicator For Chloride Titration The determination of chloride by the mohr method. Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently used titrimetric methods of analysis. The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. Titrant is used when the chloride concentration is less than. Indicator For Chloride Titration.
From dokumen.tips
(PPT) Chloride Methods Gravimetric Chloride Potentiometric titration Indicator For Chloride Titration The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. The determination of chloride by the mohr method. However, the end point is not as sharp as when a more concentrated titrant is. Discussion the mohr method uses chromate ion as an indicator in the titration of.. Indicator For Chloride Titration.
From labstellar.de
Ammonium iron (III) sulphate indicator solution for chloride titration Indicator For Chloride Titration As the silver nitrate solution is slowly. This method determines the chloride ion concentration of a solution by titration with silver nitrate. However, the end point is not as sharp as when a more concentrated titrant is. Titrant is used when the chloride concentration is less than 200 mg/l. Beside acid/base titrations, the titrimetric determination of chloride is one of. Indicator For Chloride Titration.
From slidetodoc.com
Chloride Methods q Gravimetric Chloride q Potentiometric titration Indicator For Chloride Titration Titrant is used when the chloride concentration is less than 200 mg/l. However, the end point is not as sharp as when a more concentrated titrant is. As the silver nitrate solution is slowly. Discussion the mohr method uses chromate ion as an indicator in the titration of. Beside acid/base titrations, the titrimetric determination of chloride is one of the. Indicator For Chloride Titration.
From feedwater.co.uk
Chloride Indicator and Titration kit for water testing Feedwater site Indicator For Chloride Titration As the silver nitrate solution is slowly. However, the end point is not as sharp as when a more concentrated titrant is. In the volhard method chlorides are first precipitated with excess silver nitrate, then excess silver is titrated with potassium (or. Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently used titrimetric methods of. Indicator For Chloride Titration.
From www.numerade.com
SOLVEDPotassium chromate is used as an indicator in titrations for the Indicator For Chloride Titration The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. The determination of chloride by the mohr method. As the silver nitrate solution is slowly. In the volhard method chlorides are first precipitated with excess silver nitrate, then excess silver is titrated with potassium (or. Titrant is. Indicator For Chloride Titration.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Indicator For Chloride Titration Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently used titrimetric methods of analysis. The determination of chloride by the mohr method. However, the end point is not as sharp as when a more concentrated titrant is. In the volhard method chlorides are first precipitated with excess silver nitrate, then excess silver is titrated with. Indicator For Chloride Titration.
From www.semanticscholar.org
Figure 1 from IndicatorFree Argentometric Titration for DistanceBased Indicator For Chloride Titration As the silver nitrate solution is slowly. Titrant is used when the chloride concentration is less than 200 mg/l. The determination of chloride by the mohr method. However, the end point is not as sharp as when a more concentrated titrant is. This method determines the chloride ion concentration of a solution by titration with silver nitrate. In the volhard. Indicator For Chloride Titration.
From www.wilhelmsen.com
CHLORIDE TITRATION PACK M251. Indicator For Chloride Titration Titrant is used when the chloride concentration is less than 200 mg/l. The determination of chloride by the mohr method. However, the end point is not as sharp as when a more concentrated titrant is. As the silver nitrate solution is slowly. Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently used titrimetric methods of. Indicator For Chloride Titration.
From www.scribd.com
chloride_mohr Titration Indicator For Chloride Titration Discussion the mohr method uses chromate ion as an indicator in the titration of. However, the end point is not as sharp as when a more concentrated titrant is. Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently used titrimetric methods of analysis. Titrant is used when the chloride concentration is less than 200 mg/l.. Indicator For Chloride Titration.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Indicator For Chloride Titration However, the end point is not as sharp as when a more concentrated titrant is. Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently used titrimetric methods of analysis. The determination of chloride by the mohr method. This method determines the chloride ion concentration of a solution by titration with silver nitrate. In the volhard. Indicator For Chloride Titration.
From www.researchgate.net
(PDF) IndicatorFree Argentometric Titration for DistanceBased Indicator For Chloride Titration The determination of chloride by the mohr method. Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently used titrimetric methods of analysis. Discussion the mohr method uses chromate ion as an indicator in the titration of. The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that. Indicator For Chloride Titration.
From mungfali.com
Acid Base Titration Indicator Indicator For Chloride Titration As the silver nitrate solution is slowly. In the volhard method chlorides are first precipitated with excess silver nitrate, then excess silver is titrated with potassium (or. This method determines the chloride ion concentration of a solution by titration with silver nitrate. Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently used titrimetric methods of. Indicator For Chloride Titration.
From www.numerade.com
SOLVED This method determines the chloride ion concentration of a Indicator For Chloride Titration Titrant is used when the chloride concentration is less than 200 mg/l. As the silver nitrate solution is slowly. The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. This method determines the chloride ion concentration of a solution by titration with silver nitrate. Discussion the mohr. Indicator For Chloride Titration.
From www.youtube.com
Precipitation Titrations YouTube Indicator For Chloride Titration Discussion the mohr method uses chromate ion as an indicator in the titration of. However, the end point is not as sharp as when a more concentrated titrant is. The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. Beside acid/base titrations, the titrimetric determination of chloride. Indicator For Chloride Titration.
From www.studyxapp.com
7 a mohr titration is a precipitation titration used to analyze the Indicator For Chloride Titration The determination of chloride by the mohr method. Discussion the mohr method uses chromate ion as an indicator in the titration of. This method determines the chloride ion concentration of a solution by titration with silver nitrate. In the volhard method chlorides are first precipitated with excess silver nitrate, then excess silver is titrated with potassium (or. However, the end. Indicator For Chloride Titration.
From www.reddit.com
When you get into a rhythm with your sodium chloride titrations. A Indicator For Chloride Titration This method determines the chloride ion concentration of a solution by titration with silver nitrate. As the silver nitrate solution is slowly. However, the end point is not as sharp as when a more concentrated titrant is. Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently used titrimetric methods of analysis. Titrant is used when. Indicator For Chloride Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Indicator For Chloride Titration The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. The determination of chloride by the mohr method. Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently used titrimetric methods of analysis. As the silver nitrate solution is slowly. Titrant is used. Indicator For Chloride Titration.
From ar.inspiredpencil.com
Titration Diagram Indicator For Chloride Titration In the volhard method chlorides are first precipitated with excess silver nitrate, then excess silver is titrated with potassium (or. However, the end point is not as sharp as when a more concentrated titrant is. The concentration of chloride ions is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate. This method. Indicator For Chloride Titration.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Indicator For Chloride Titration Titrant is used when the chloride concentration is less than 200 mg/l. This method determines the chloride ion concentration of a solution by titration with silver nitrate. Beside acid/base titrations, the titrimetric determination of chloride is one of the most frequently used titrimetric methods of analysis. The determination of chloride by the mohr method. In the volhard method chlorides are. Indicator For Chloride Titration.
From www.numerade.com
SOLVED In a Mohr titration of chloride with silver nitrate, an error Indicator For Chloride Titration Titrant is used when the chloride concentration is less than 200 mg/l. As the silver nitrate solution is slowly. Discussion the mohr method uses chromate ion as an indicator in the titration of. This method determines the chloride ion concentration of a solution by titration with silver nitrate. Beside acid/base titrations, the titrimetric determination of chloride is one of the. Indicator For Chloride Titration.