What Is The Body's Buffer System . Proteins assist with intracellular ph regulation. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. The phosphate buffer system, while present globally, is important for the regulation of urine ph. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. Chemical buffers, such as bicarbonate and ammonia, help keep the blood’s ph in the narrow range that is compatible with life. Various buffer systems exist in body fluids (see table) to minimise the effects on ph of the addition or removal of acid from them. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Buffers are solutions that contain a weak acid and its a conjugate base;
from www.slideserve.com
Buffers are solutions that contain a weak acid and its a conjugate base; Proteins assist with intracellular ph regulation. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. The phosphate buffer system, while present globally, is important for the regulation of urine ph. Chemical buffers, such as bicarbonate and ammonia, help keep the blood’s ph in the narrow range that is compatible with life. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Various buffer systems exist in body fluids (see table) to minimise the effects on ph of the addition or removal of acid from them.
PPT Water & Buffers PowerPoint Presentation, free download ID3886735
What Is The Body's Buffer System The phosphate buffer system, while present globally, is important for the regulation of urine ph. Proteins assist with intracellular ph regulation. The phosphate buffer system, while present globally, is important for the regulation of urine ph. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. Buffers are solutions that contain a weak acid and its a conjugate base; Various buffer systems exist in body fluids (see table) to minimise the effects on ph of the addition or removal of acid from them. Chemical buffers, such as bicarbonate and ammonia, help keep the blood’s ph in the narrow range that is compatible with life.
From www.slideserve.com
PPT Unit Five The Body Fluids and Kidneys PowerPoint Presentation, free download ID3308641 What Is The Body's Buffer System Proteins assist with intracellular ph regulation. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. The phosphate buffer system, while present globally, is important for the regulation of urine ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers.. What Is The Body's Buffer System.
From www.studocu.com
Biological Buffer Systems BIOLOGICAL BUFFER SYSTEMS Almost every biological process is Studocu What Is The Body's Buffer System Proteins assist with intracellular ph regulation. Buffers are solutions that contain a weak acid and its a conjugate base; Chemical buffers, such as bicarbonate and ammonia, help keep the blood’s ph in the narrow range that is compatible with life. The phosphate buffer system, while present globally, is important for the regulation of urine ph. A variety of buffering systems. What Is The Body's Buffer System.
From www.slideserve.com
PPT Integrative Physiology PowerPoint Presentation, free download ID2426335 What Is The Body's Buffer System The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. Chemical buffers, such as bicarbonate and ammonia, help keep the blood’s ph in the narrow range that is compatible with. What Is The Body's Buffer System.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID2127603 What Is The Body's Buffer System The phosphate buffer system, while present globally, is important for the regulation of urine ph. Various buffer systems exist in body fluids (see table) to minimise the effects on ph of the addition or removal of acid from them. Proteins assist with intracellular ph regulation. Buffers are solutions that contain a weak acid and its a conjugate base; Chemical buffers,. What Is The Body's Buffer System.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation ID257615 What Is The Body's Buffer System A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. Chemical buffers, such as bicarbonate and ammonia, help keep the blood’s ph in the narrow range that is compatible with life. The phosphate buffer system, while present globally, is important for the regulation of urine ph. Buffers are. What Is The Body's Buffer System.
From joirryihd.blob.core.windows.net
What Are The Buffers In The Body at Frank Stookey blog What Is The Body's Buffer System Chemical buffers, such as bicarbonate and ammonia, help keep the blood’s ph in the narrow range that is compatible with life. Various buffer systems exist in body fluids (see table) to minimise the effects on ph of the addition or removal of acid from them. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic. What Is The Body's Buffer System.
From www.slideshare.net
Buffer system What Is The Body's Buffer System A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. The phosphate buffer system, while present globally, is important for the regulation of urine ph. Proteins assist with intracellular ph regulation. Chemical buffers, such as bicarbonate and ammonia, help keep the blood’s ph in the narrow range. What Is The Body's Buffer System.
From ridtpoof-lano.blogspot.com
What Is The Most Powerful Buffer System In The Body Maria Ma Coiffure What Is The Body's Buffer System Buffers are solutions that contain a weak acid and its a conjugate base; Various buffer systems exist in body fluids (see table) to minimise the effects on ph of the addition or removal of acid from them. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Chemical buffers, such as bicarbonate and. What Is The Body's Buffer System.
From ppt-online.org
Disorders of metabolism. (Subject 9) презентация онлайн What Is The Body's Buffer System The phosphate buffer system, while present globally, is important for the regulation of urine ph. Buffers are solutions that contain a weak acid and its a conjugate base; A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. A variety of buffering systems permits blood and other bodily. What Is The Body's Buffer System.
From www.slideserve.com
PPT Physiological system of blood. Functional importance of blood plasma components What Is The Body's Buffer System The phosphate buffer system, while present globally, is important for the regulation of urine ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Proteins assist with intracellular ph regulation. Chemical buffers, such as bicarbonate and ammonia, help keep the blood’s ph in the narrow range that is compatible with life. A. What Is The Body's Buffer System.
From studylib.net
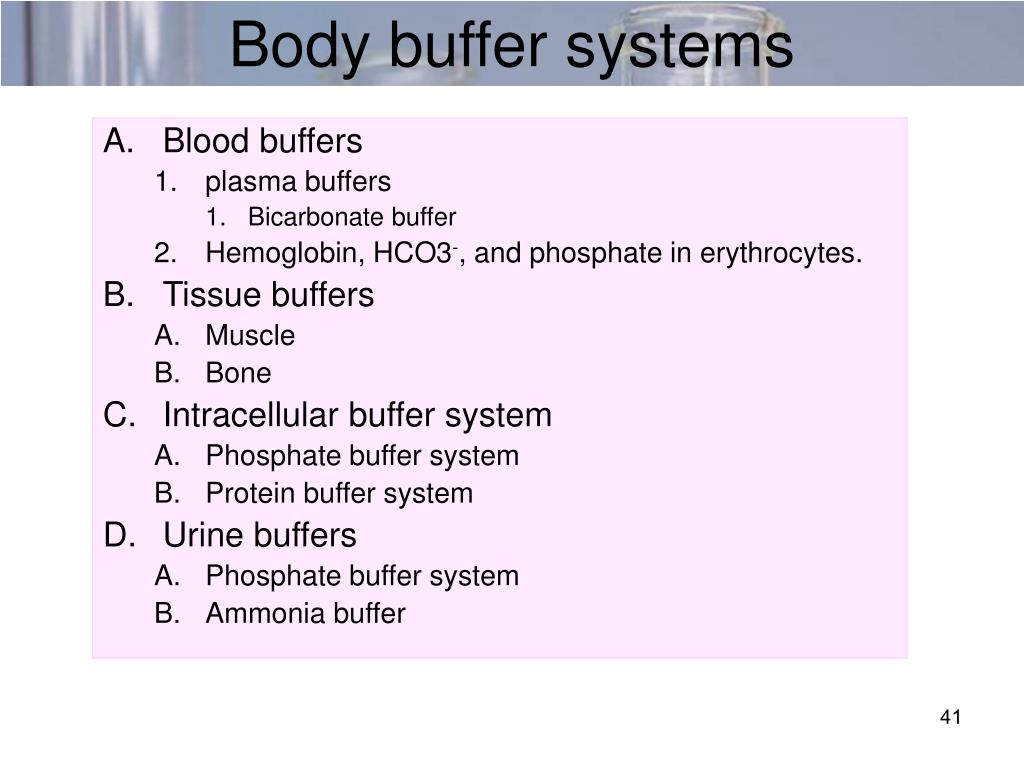
Body Buffer Systems What Is The Body's Buffer System The phosphate buffer system, while present globally, is important for the regulation of urine ph. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. Proteins assist with intracellular ph regulation. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph. What Is The Body's Buffer System.
From www.youtube.com
Buffer action in the blood YouTube What Is The Body's Buffer System A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. Chemical buffers, such as bicarbonate and ammonia, help keep the blood’s ph in the narrow range that is compatible with life. Various buffer systems exist in body fluids (see table) to minimise the effects on ph of the. What Is The Body's Buffer System.
From www.slideshare.net
Buffer system What Is The Body's Buffer System The phosphate buffer system, while present globally, is important for the regulation of urine ph. Proteins assist with intracellular ph regulation. Buffers are solutions that contain a weak acid and its a conjugate base; A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. Chemical buffers, such as. What Is The Body's Buffer System.
From www.slideshare.net
Acid base balance What Is The Body's Buffer System Chemical buffers, such as bicarbonate and ammonia, help keep the blood’s ph in the narrow range that is compatible with life. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. What Is The Body's Buffer System.
From studyloadstones.z21.web.core.windows.net
Why Are Buffers Important To Homeostasis What Is The Body's Buffer System A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. Chemical buffers, such as bicarbonate and ammonia, help keep the blood’s ph in the narrow range that is compatible with life. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid. What Is The Body's Buffer System.
From www.pinterest.com
Physiology Blood Buffer System Behrouz Human body facts, Biochemistry, Physiology What Is The Body's Buffer System The phosphate buffer system, while present globally, is important for the regulation of urine ph. Chemical buffers, such as bicarbonate and ammonia, help keep the blood’s ph in the narrow range that is compatible with life. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Proteins assist with intracellular ph regulation. Various. What Is The Body's Buffer System.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID2127603 What Is The Body's Buffer System The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Chemical buffers, such as bicarbonate and ammonia, help keep the blood’s ph in the narrow range that is compatible with life. The phosphate buffer system, while present globally, is important for the regulation of urine ph. Proteins assist with intracellular ph regulation. A. What Is The Body's Buffer System.
From www.slideserve.com
PPT Acids and Bases and Buffers PowerPoint Presentation, free download ID4769309 What Is The Body's Buffer System Buffers are solutions that contain a weak acid and its a conjugate base; Chemical buffers, such as bicarbonate and ammonia, help keep the blood’s ph in the narrow range that is compatible with life. The phosphate buffer system, while present globally, is important for the regulation of urine ph. A variety of buffering systems permits blood and other bodily fluids. What Is The Body's Buffer System.
From www.studypool.com
SOLUTION Body buffer system Studypool What Is The Body's Buffer System The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Proteins assist with intracellular ph regulation. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow. What Is The Body's Buffer System.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID2127603 What Is The Body's Buffer System Various buffer systems exist in body fluids (see table) to minimise the effects on ph of the addition or removal of acid from them. Buffers are solutions that contain a weak acid and its a conjugate base; A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. A. What Is The Body's Buffer System.
From labpedia.net
Acidbase Balance Part 3 Respiratory Acidosis and Respiratory Alkalosis What Is The Body's Buffer System A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. Proteins assist with intracellular ph regulation. The buffer systems functioning in blood plasma include plasma. What Is The Body's Buffer System.
From www.slideshare.net
Buffer system What Is The Body's Buffer System Chemical buffers, such as bicarbonate and ammonia, help keep the blood’s ph in the narrow range that is compatible with life. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. Buffers are solutions that contain a weak acid and its a conjugate base; The phosphate buffer. What Is The Body's Buffer System.
From www.slideserve.com
PPT Homeostasis PowerPoint Presentation, free download ID1138529 What Is The Body's Buffer System Chemical buffers, such as bicarbonate and ammonia, help keep the blood’s ph in the narrow range that is compatible with life. Proteins assist with intracellular ph regulation. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The phosphate buffer system, while present globally, is important for the regulation of urine ph. A. What Is The Body's Buffer System.
From www.pinterest.ca
bicarbonate buffer system, example of multiple equilibria Teaching chemistry, Medical school What Is The Body's Buffer System A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. The phosphate buffer system, while present globally, is important for the regulation of urine ph. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of.. What Is The Body's Buffer System.
From www.slideserve.com
PPT Fluid, Electrolyte, and AcidBase Balance PowerPoint Presentation ID297131 What Is The Body's Buffer System The phosphate buffer system, while present globally, is important for the regulation of urine ph. Various buffer systems exist in body fluids (see table) to minimise the effects on ph of the addition or removal of acid from them. Chemical buffers, such as bicarbonate and ammonia, help keep the blood’s ph in the narrow range that is compatible with life.. What Is The Body's Buffer System.
From www.slideserve.com
PPT AcidBase Balance PowerPoint Presentation, free download ID2821926 What Is The Body's Buffer System A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. Buffers are solutions that contain a weak acid and its a conjugate base; The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Chemical buffers, such as bicarbonate and ammonia,. What Is The Body's Buffer System.
From www.slideserve.com
PPT Buffer Systems of the Body PowerPoint Presentation, free download ID4264848 What Is The Body's Buffer System Chemical buffers, such as bicarbonate and ammonia, help keep the blood’s ph in the narrow range that is compatible with life. Various buffer systems exist in body fluids (see table) to minimise the effects on ph of the addition or removal of acid from them. The phosphate buffer system, while present globally, is important for the regulation of urine ph.. What Is The Body's Buffer System.
From www.slideserve.com
PPT Renal Physiology PowerPoint Presentation, free download ID5632772 What Is The Body's Buffer System The phosphate buffer system, while present globally, is important for the regulation of urine ph. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. Proteins assist with intracellular ph regulation. Various buffer systems exist in body fluids (see table) to minimise the effects on ph of the. What Is The Body's Buffer System.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Presentation ID5857540 What Is The Body's Buffer System A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. Buffers are solutions that contain a weak acid and its a conjugate base; The phosphate. What Is The Body's Buffer System.
From www.slideserve.com
PPT Buffer Systems of the Body PowerPoint Presentation, free download ID4264848 What Is The Body's Buffer System A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. Proteins assist with intracellular ph regulation. The phosphate buffer system, while present globally, is important. What Is The Body's Buffer System.
From www.slideserve.com
PPT Chapter 27 Fluid, Electrolyte and Acidbase Balance PowerPoint Presentation ID2687569 What Is The Body's Buffer System Proteins assist with intracellular ph regulation. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. Various buffer systems exist in body fluids (see table). What Is The Body's Buffer System.
From www.slideserve.com
PPT Water & Buffers PowerPoint Presentation, free download ID3886735 What Is The Body's Buffer System The phosphate buffer system, while present globally, is important for the regulation of urine ph. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of.. What Is The Body's Buffer System.
From ridtpoof-lano.blogspot.com
What Is The Most Powerful Buffer System In The Body Maria Ma Coiffure What Is The Body's Buffer System Proteins assist with intracellular ph regulation. The phosphate buffer system, while present globally, is important for the regulation of urine ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. What Is The Body's Buffer System.
From www.slideserve.com
PPT Unit Five The Body Fluids and Kidneys PowerPoint Presentation, free download ID3308641 What Is The Body's Buffer System A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. The phosphate buffer system, while present globally, is important for the regulation of urine ph. Chemical buffers, such as bicarbonate and ammonia, help keep the blood’s ph in the narrow range that is compatible with life. Proteins assist. What Is The Body's Buffer System.
From www.slideserve.com
PPT 1. Body Fluid Compartments PowerPoint Presentation, free download ID6851983 What Is The Body's Buffer System A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. Proteins assist with intracellular ph regulation. The phosphate buffer system, while present globally, is important for the regulation of urine ph. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range,. What Is The Body's Buffer System.