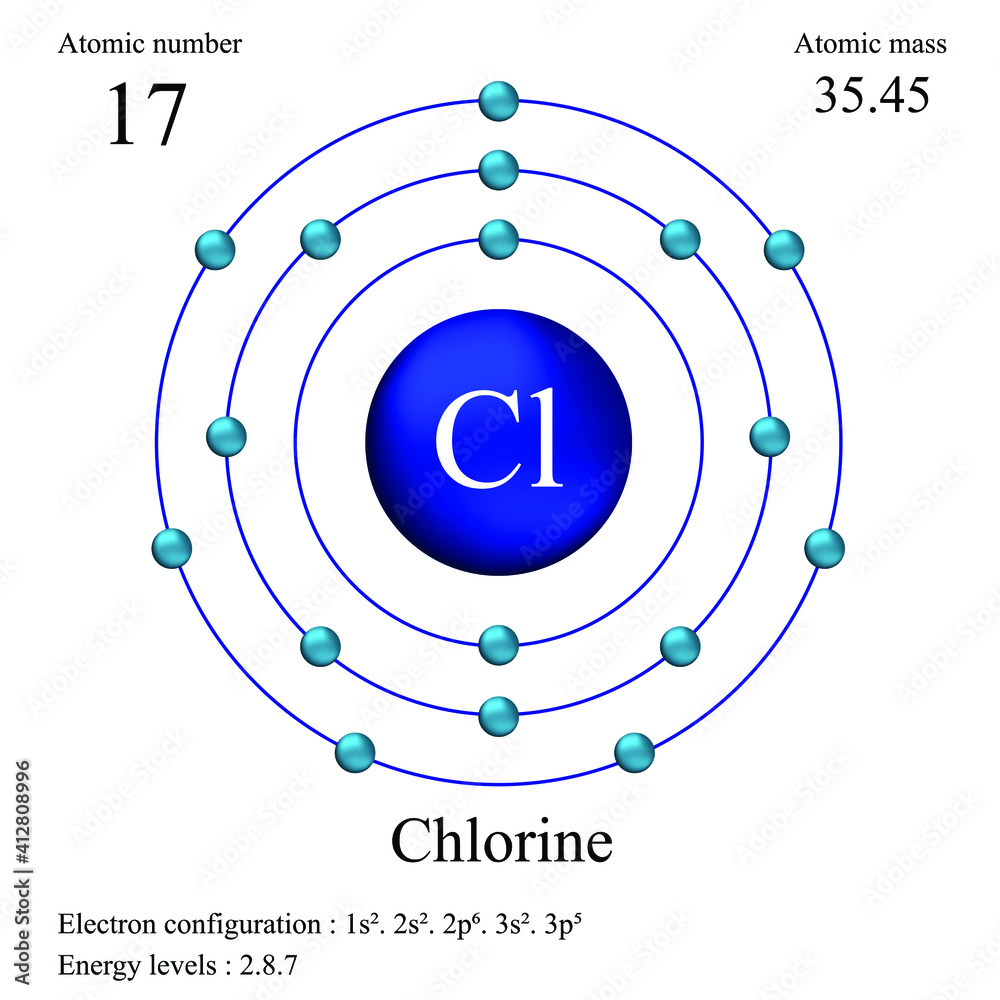
Chlorine 35 Mass . Chlorine has seventeen protons and eighteen neutrons in its nucleus, and seventeen electrons in three shells. it has an atomic weight of 35.450 and a mass number of 35. Isotope mass / da natural abundance (atom %) nuclear spin (i) magnetic moment (μ/μ n) 35 cl:. 34.9688527(3) u (atomic weight of. atomic mass of chlorine is 35.453 u. 35 cl or 35 17 cl mass number a: The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. It is located in group seventeen, period three. further data for radioisotopes (radioactive isotopes) of chlorine are listed (including any which occur naturally) below. 17 (= number of protons) neutrons n: 35 (= number of nucleons) atomic number z: Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. If a single value of the atomic mass is needed, use 35.4527. The atomic mass is the mass of an atom. 35.457] iupac guidelines to reflect the physical and chemical history of the magnesium sample.
from stock.adobe.com
34.9688527(3) u (atomic weight of. If a single value of the atomic mass is needed, use 35.4527. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. 17 (= number of protons) neutrons n: The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. Chlorine has seventeen protons and eighteen neutrons in its nucleus, and seventeen electrons in three shells. Isotope mass / da natural abundance (atom %) nuclear spin (i) magnetic moment (μ/μ n) 35 cl:. The atomic mass is the mass of an atom. atomic mass of chlorine is 35.453 u. further data for radioisotopes (radioactive isotopes) of chlorine are listed (including any which occur naturally) below.
Chlorine atomic structure has atomic number, atomic mass, electron
Chlorine 35 Mass name of the isotope: atomic mass of chlorine is 35.453 u. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. The atomic mass is the mass of an atom. 34.9688527(3) u (atomic weight of. further data for radioisotopes (radioactive isotopes) of chlorine are listed (including any which occur naturally) below. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. If a single value of the atomic mass is needed, use 35.4527. Chlorine has seventeen protons and eighteen neutrons in its nucleus, and seventeen electrons in three shells. 17 (= number of protons) neutrons n: 35.457] iupac guidelines to reflect the physical and chemical history of the magnesium sample. name of the isotope: 35 cl or 35 17 cl mass number a: Isotope mass / da natural abundance (atom %) nuclear spin (i) magnetic moment (μ/μ n) 35 cl:. 35 (= number of nucleons) atomic number z: It is located in group seventeen, period three.
From elchoroukhost.net
Chlorine Periodic Table Mass Number Elcho Table Chlorine 35 Mass 35.457] iupac guidelines to reflect the physical and chemical history of the magnesium sample. Isotope mass / da natural abundance (atom %) nuclear spin (i) magnetic moment (μ/μ n) 35 cl:. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. Chlorine has seventeen protons and eighteen neutrons in its nucleus, and seventeen electrons in three shells. 17 (=. Chlorine 35 Mass.
From greekchlist.weebly.com
Chlorine atomic mass greekchlist Chlorine 35 Mass The atomic mass is the mass of an atom. 35 (= number of nucleons) atomic number z: further data for radioisotopes (radioactive isotopes) of chlorine are listed (including any which occur naturally) below. 17 (= number of protons) neutrons n: Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. it has an atomic weight of 35.450. Chlorine 35 Mass.
From brainly.in
Atomic mass of chlorine is 35.5. It has two isotopes of atomic mass 35 Chlorine 35 Mass atomic mass of chlorine is 35.453 u. 35.457] iupac guidelines to reflect the physical and chemical history of the magnesium sample. It is located in group seventeen, period three. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. Sources, facts, uses,. Chlorine 35 Mass.
From www.chemistrylearner.com
Chlorine Facts, Symbol, Discovery, Properties, Uses Chlorine 35 Mass The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. 34.9688527(3) u (atomic weight of. 35 cl or 35 17 cl mass number a: 35.457] iupac guidelines to reflect the physical and chemical history of the magnesium sample. further data for radioisotopes. Chlorine 35 Mass.
From askfilo.com
Average atomic mass of chlorine is 35.5u. It has two isotopes of atomic m.. Chlorine 35 Mass It is located in group seventeen, period three. further data for radioisotopes (radioactive isotopes) of chlorine are listed (including any which occur naturally) below. 35 cl or 35 17 cl mass number a: Isotope mass / da natural abundance (atom %) nuclear spin (i) magnetic moment (μ/μ n) 35 cl:. 35.457] iupac guidelines to reflect the physical and chemical. Chlorine 35 Mass.
From www.livescience.com
Facts About Chlorine Live Science Chlorine 35 Mass Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. 35.457] iupac guidelines to reflect the physical and chemical history of the magnesium sample. it has an atomic weight of 35.450 and a mass number of 35. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a. Chlorine 35 Mass.
From science4fun.info
Chlorine Element (Properties, Uses, and Facts) Science4Fun Chlorine 35 Mass name of the isotope: If a single value of the atomic mass is needed, use 35.4527. atomic mass of chlorine is 35.453 u. The atomic mass is the mass of an atom. 35 cl or 35 17 cl mass number a: 34.9688527(3) u (atomic weight of. Chlorine has seventeen protons and eighteen neutrons in its nucleus, and seventeen. Chlorine 35 Mass.
From www.slideserve.com
PPT Stoichiometry PowerPoint Presentation, free download ID5435894 Chlorine 35 Mass 17 (= number of protons) neutrons n: 35.457] iupac guidelines to reflect the physical and chemical history of the magnesium sample. 34.9688527(3) u (atomic weight of. 35 cl or 35 17 cl mass number a: If a single value of the atomic mass is needed, use 35.4527. 35 (= number of nucleons) atomic number z: atomic mass of chlorine. Chlorine 35 Mass.
From brainly.com
Chlorine has two isotopes, 35Cl and 37Cl; 75.77 of chlorine is 35Cl Chlorine 35 Mass If a single value of the atomic mass is needed, use 35.4527. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. 35 cl or 35 17 cl mass number a: The atomic mass is the mass of an atom. 35.457] iupac guidelines to reflect the physical and chemical history of the magnesium sample. Chlorine has seventeen protons and. Chlorine 35 Mass.
From byjus.com
40. Atomic weight of chlorine is 35.5.It has two isotopes of atomic Chlorine 35 Mass Isotope mass / da natural abundance (atom %) nuclear spin (i) magnetic moment (μ/μ n) 35 cl:. Chlorine has seventeen protons and eighteen neutrons in its nucleus, and seventeen electrons in three shells. it has an atomic weight of 35.450 and a mass number of 35. 35 (= number of nucleons) atomic number z: The atomic mass or relative. Chlorine 35 Mass.
From www.animalia-life.club
Chlorine Model Project Chlorine 35 Mass Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. name of the isotope: The atomic mass is the mass of an atom. Isotope mass / da natural abundance (atom %) nuclear spin (i) magnetic moment (μ/μ n) 35 cl:. 17 (= number of protons) neutrons n: further data for radioisotopes (radioactive isotopes) of chlorine are listed. Chlorine 35 Mass.
From www.vecteezy.com
Chlorine chemical element Chemical symbol with atomic number and atomic Chlorine 35 Mass 17 (= number of protons) neutrons n: 34.9688527(3) u (atomic weight of. 35 (= number of nucleons) atomic number z: Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. atomic mass of chlorine is 35.453 u. Chlorine has seventeen protons and eighteen neutrons in its nucleus, and seventeen electrons in three shells. it has an atomic. Chlorine 35 Mass.
From www.youtube.com
Average Atomic Mass Calculation (Chlorine) YouTube Chlorine 35 Mass 35.457] iupac guidelines to reflect the physical and chemical history of the magnesium sample. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. 35 (= number of nucleons) atomic number z: atomic mass of chlorine is 35.453 u. Chlorine has seventeen protons and eighteen neutrons in its nucleus, and seventeen electrons in three shells. If a single. Chlorine 35 Mass.
From ar.inspiredpencil.com
Chlorine Molecule Model Chlorine 35 Mass The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. It is located in group seventeen, period three. Isotope mass / da natural abundance (atom %) nuclear spin (i) magnetic moment (μ/μ. Chlorine 35 Mass.
From www.buyisotope.com
Chlorine35, Chlorine35 Isotope, Enriched Chlorine35 Chlorine 35 Mass Chlorine has seventeen protons and eighteen neutrons in its nucleus, and seventeen electrons in three shells. It is located in group seventeen, period three. 35.457] iupac guidelines to reflect the physical and chemical history of the magnesium sample. Isotope mass / da natural abundance (atom %) nuclear spin (i) magnetic moment (μ/μ n) 35 cl:. name of the isotope:. Chlorine 35 Mass.
From www.showme.com
Relative abundancies of Chlorine from a mass spectrometer Science Chlorine 35 Mass further data for radioisotopes (radioactive isotopes) of chlorine are listed (including any which occur naturally) below. 35 (= number of nucleons) atomic number z: 35 cl or 35 17 cl mass number a: name of the isotope: If a single value of the atomic mass is needed, use 35.4527. 35.457] iupac guidelines to reflect the physical and chemical. Chlorine 35 Mass.
From www.slideserve.com
PPT 1. Atomic Structure PowerPoint Presentation, free download ID Chlorine 35 Mass further data for radioisotopes (radioactive isotopes) of chlorine are listed (including any which occur naturally) below. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. 35 cl or 35 17 cl mass number a: atomic mass of chlorine is 35.453. Chlorine 35 Mass.
From byjus.com
60. If the average atomic mass of chlorine is 35.5, then find the Chlorine 35 Mass it has an atomic weight of 35.450 and a mass number of 35. Isotope mass / da natural abundance (atom %) nuclear spin (i) magnetic moment (μ/μ n) 35 cl:. name of the isotope: The atomic mass is the mass of an atom. further data for radioisotopes (radioactive isotopes) of chlorine are listed (including any which occur. Chlorine 35 Mass.
From stock.adobe.com
Chlorine atomic structure has atomic number, atomic mass, electron Chlorine 35 Mass it has an atomic weight of 35.450 and a mass number of 35. If a single value of the atomic mass is needed, use 35.4527. 35.457] iupac guidelines to reflect the physical and chemical history of the magnesium sample. atomic mass of chlorine is 35.453 u. 35 (= number of nucleons) atomic number z: The atomic mass is. Chlorine 35 Mass.
From www.instantuition.com
Mass Spectrometry of Chlorine O Level Chemistry Chlorine 35 Mass It is located in group seventeen, period three. further data for radioisotopes (radioactive isotopes) of chlorine are listed (including any which occur naturally) below. atomic mass of chlorine is 35.453 u. The atomic mass is the mass of an atom. 35.457] iupac guidelines to reflect the physical and chemical history of the magnesium sample. Isotope mass / da. Chlorine 35 Mass.
From www.britannica.com
Chlorine Uses, Properties, & Facts Britannica Chlorine 35 Mass The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. atomic mass of chlorine is 35.453 u. 35.457] iupac guidelines to reflect the physical and chemical history of the magnesium sample. 35 cl or 35 17 cl mass number a: it. Chlorine 35 Mass.
From www.coursehero.com
[Solved] Chlorine has two naturally occurring isotopes Chlorine 35 Chlorine 35 Mass Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. further data for radioisotopes (radioactive isotopes) of chlorine are listed (including any which occur naturally) below. The atomic mass is the mass of an atom. It is located in group seventeen, period three. The atomic mass or relative isotopic mass refers to the mass of a single particle,. Chlorine 35 Mass.
From resnurse.weebly.com
Chlorine atomic mass resnurse Chlorine 35 Mass Chlorine has seventeen protons and eighteen neutrons in its nucleus, and seventeen electrons in three shells. It is located in group seventeen, period three. name of the isotope: If a single value of the atomic mass is needed, use 35.4527. 35.457] iupac guidelines to reflect the physical and chemical history of the magnesium sample. The atomic mass or relative. Chlorine 35 Mass.
From www.toppr.com
The isotopes of chlorine with mass number 35 and 37 exist in the ratio of Chlorine 35 Mass 35.457] iupac guidelines to reflect the physical and chemical history of the magnesium sample. name of the isotope: The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. it has an atomic weight of 35.450 and a mass number of 35.. Chlorine 35 Mass.
From www.toppr.com
Chlorine has two naturally occurring isotopes, ^35Cl and ^37Cl . If the Chlorine 35 Mass it has an atomic weight of 35.450 and a mass number of 35. If a single value of the atomic mass is needed, use 35.4527. further data for radioisotopes (radioactive isotopes) of chlorine are listed (including any which occur naturally) below. The atomic mass is the mass of an atom. 35 (= number of nucleons) atomic number z:. Chlorine 35 Mass.
From brainly.in
Explain why chlorine always has relative atomic mass of about 35.5u Chlorine 35 Mass If a single value of the atomic mass is needed, use 35.4527. It is located in group seventeen, period three. 35 (= number of nucleons) atomic number z: further data for radioisotopes (radioactive isotopes) of chlorine are listed (including any which occur naturally) below. 35.457] iupac guidelines to reflect the physical and chemical history of the magnesium sample. 34.9688527(3). Chlorine 35 Mass.
From www.toppr.com
Atomic mass of chlorine is 35.5. It has two isotopes of atomic mass 35 Chlorine 35 Mass The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. further data for radioisotopes (radioactive isotopes) of chlorine are listed (including any which occur naturally) below. 35 cl or 35 17. Chlorine 35 Mass.
From www.teachoo.com
Isotopes and Isobars Definition, Uses and Difference Teachoo Chlorine 35 Mass atomic mass of chlorine is 35.453 u. 35.457] iupac guidelines to reflect the physical and chemical history of the magnesium sample. 34.9688527(3) u (atomic weight of. The atomic mass is the mass of an atom. further data for radioisotopes (radioactive isotopes) of chlorine are listed (including any which occur naturally) below. Sources, facts, uses, scarcity (sri), podcasts, alchemical. Chlorine 35 Mass.
From www.toppr.com
A naturally occurring mixture of chlorine has the following fractional Chlorine 35 Mass further data for radioisotopes (radioactive isotopes) of chlorine are listed (including any which occur naturally) below. If a single value of the atomic mass is needed, use 35.4527. Chlorine has seventeen protons and eighteen neutrons in its nucleus, and seventeen electrons in three shells. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. The atomic mass is. Chlorine 35 Mass.
From byjus.com
cl 35 and cl 37 are two isotopes of chlorine . if average atomic mass Chlorine 35 Mass atomic mass of chlorine is 35.453 u. 17 (= number of protons) neutrons n: name of the isotope: Isotope mass / da natural abundance (atom %) nuclear spin (i) magnetic moment (μ/μ n) 35 cl:. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. If a single value of the atomic mass is needed, use 35.4527.. Chlorine 35 Mass.
From www.dreamstime.com
Diagram Representation of the Element Chlorine Stock Illustration Chlorine 35 Mass If a single value of the atomic mass is needed, use 35.4527. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. 35 (= number of nucleons) atomic number z: 17 (= number of protons) neutrons n: atomic mass of chlorine is. Chlorine 35 Mass.
From www.chegg.com
Solved There are two naturally occurring isotopes of Chlorine 35 Mass it has an atomic weight of 35.450 and a mass number of 35. The atomic mass is the mass of an atom. If a single value of the atomic mass is needed, use 35.4527. atomic mass of chlorine is 35.453 u. Chlorine has seventeen protons and eighteen neutrons in its nucleus, and seventeen electrons in three shells. 35.457]. Chlorine 35 Mass.
From www.pinterest.com
Why is the relative atomic mass of chlorine 35.5? Relative atomic Chlorine 35 Mass The atomic mass is the mass of an atom. Chlorine has seventeen protons and eighteen neutrons in its nucleus, and seventeen electrons in three shells. It is located in group seventeen, period three. 35 cl or 35 17 cl mass number a: The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is. Chlorine 35 Mass.
From www.nuclear-power.com
Chlorine Atomic Number Atomic Mass Density of Chlorine nuclear Chlorine 35 Mass 35.457] iupac guidelines to reflect the physical and chemical history of the magnesium sample. name of the isotope: 17 (= number of protons) neutrons n: it has an atomic weight of 35.450 and a mass number of 35. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. The atomic mass or relative isotopic mass refers to. Chlorine 35 Mass.
From www.chegg.com
Solved Chlorine Has An Atomic Mass Of 35 And An Atomic Nu... Chlorine 35 Mass it has an atomic weight of 35.450 and a mass number of 35. further data for radioisotopes (radioactive isotopes) of chlorine are listed (including any which occur naturally) below. 35 cl or 35 17 cl mass number a: Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. It is located in group seventeen, period three. 34.9688527(3). Chlorine 35 Mass.