How To Calculate Halfway Point Titration . Ma is the molarity of the acid, while mb is the molarity of the base. how to find the half equivalence point knowing the ph, molarity, titrant added at equivalence point? — number of h + ions. This a fairly straightforward and simple question, however i have found many different. the half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. ma × va = mb × vb. you can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. Va and vb are the volumes of the acid and base, respectively. An explanation and two examples of the halfway point in a titration. Suppose that a titration is performed and 20.70ml of 0.500mnaoh is. the ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the. 7.1k views 4 years ago.
from www.youtube.com
An explanation and two examples of the halfway point in a titration. the half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. ma × va = mb × vb. the ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the. Ma is the molarity of the acid, while mb is the molarity of the base. Va and vb are the volumes of the acid and base, respectively. Suppose that a titration is performed and 20.70ml of 0.500mnaoh is. — number of h + ions. you can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. 7.1k views 4 years ago.
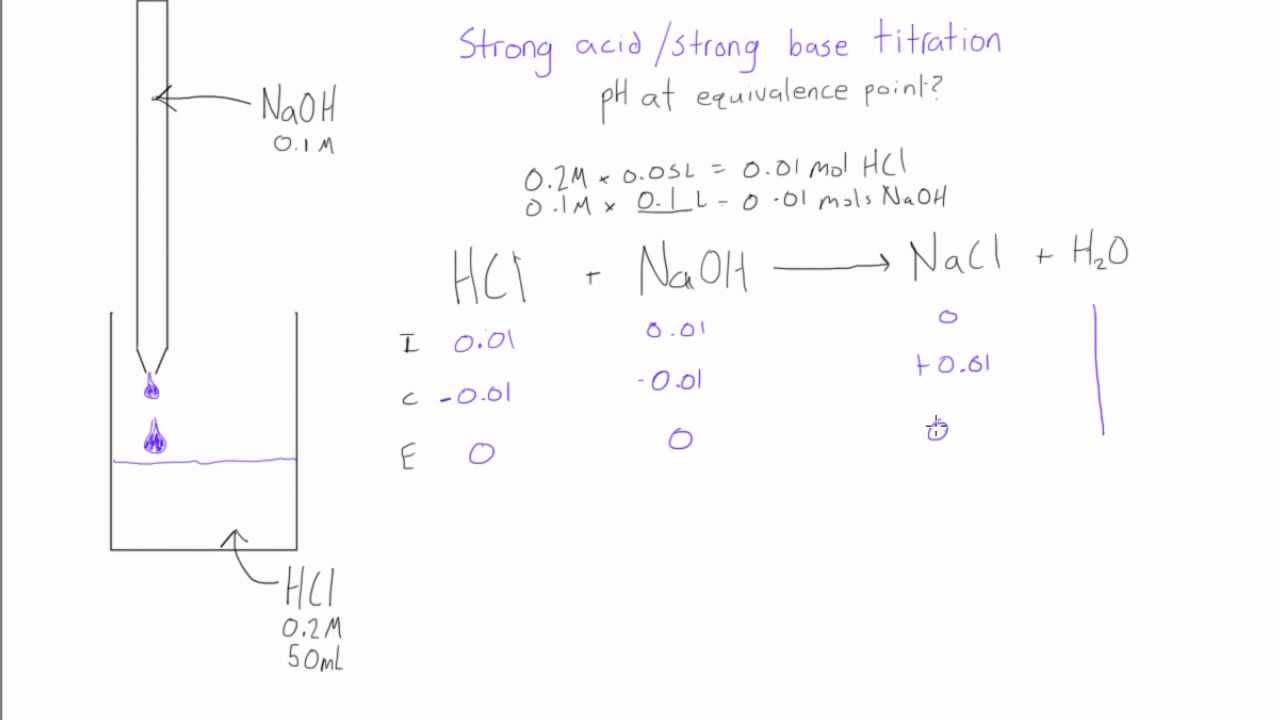
Strong acid / strong base titration pH at equivalence point YouTube
How To Calculate Halfway Point Titration the half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. 7.1k views 4 years ago. how to find the half equivalence point knowing the ph, molarity, titrant added at equivalence point? Ma is the molarity of the acid, while mb is the molarity of the base. the ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the. ma × va = mb × vb. — number of h + ions. This a fairly straightforward and simple question, however i have found many different. An explanation and two examples of the halfway point in a titration. the half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. Suppose that a titration is performed and 20.70ml of 0.500mnaoh is. Va and vb are the volumes of the acid and base, respectively. you can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is.
From www.myxxgirl.com
Titration Titrimetry Or Volumetric Analysis A Burette And Erlenmeyer How To Calculate Halfway Point Titration Suppose that a titration is performed and 20.70ml of 0.500mnaoh is. Ma is the molarity of the acid, while mb is the molarity of the base. This a fairly straightforward and simple question, however i have found many different. 7.1k views 4 years ago. the ph at the midpoint, the point halfway on the titration curve to the equivalence. How To Calculate Halfway Point Titration.
From www.solutionspile.com
[Solved] Calculate the ( mathrm{pH} ) at the halfway p How To Calculate Halfway Point Titration the half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. Suppose that a titration is performed and 20.70ml of 0.500mnaoh is. you can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial. How To Calculate Halfway Point Titration.
From www.chegg.com
Solved Calculate the pH at the halfway point and at the How To Calculate Halfway Point Titration the half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. An explanation and two examples of the halfway point in a titration. 7.1k views 4 years ago. Va and vb are the volumes of the acid and base, respectively. the ph at the midpoint, the point. How To Calculate Halfway Point Titration.
From philschatz.com
AcidBase Titrations · Chemistry How To Calculate Halfway Point Titration — number of h + ions. An explanation and two examples of the halfway point in a titration. Va and vb are the volumes of the acid and base, respectively. the ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the. the half equivalence point is. How To Calculate Halfway Point Titration.
From www.chegg.com
Solved Calculate the pH at the halfway point and at the How To Calculate Halfway Point Titration This a fairly straightforward and simple question, however i have found many different. — number of h + ions. 7.1k views 4 years ago. the ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the. how to find the half equivalence point knowing the ph, molarity,. How To Calculate Halfway Point Titration.
From www.chegg.com
Solved 7. Based On The Curve Below For The Titration Of 0... How To Calculate Halfway Point Titration Suppose that a titration is performed and 20.70ml of 0.500mnaoh is. Ma is the molarity of the acid, while mb is the molarity of the base. you can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. 7.1k views 4 years ago. . How To Calculate Halfway Point Titration.
From www.hotzxgirl.com
Titration Curves Of Amino Acids Food Science Toolbox Hot Sex Picture How To Calculate Halfway Point Titration 7.1k views 4 years ago. This a fairly straightforward and simple question, however i have found many different. Ma is the molarity of the acid, while mb is the molarity of the base. Suppose that a titration is performed and 20.70ml of 0.500mnaoh is. — number of h + ions. ma × va = mb × vb. Va and. How To Calculate Halfway Point Titration.
From www.numerade.com
SOLVED 7/6 points ZumChemp? E.069. Calculate the pH at the halfway How To Calculate Halfway Point Titration An explanation and two examples of the halfway point in a titration. the half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. Ma is the molarity of the acid, while mb is the molarity of the base. 7.1k views 4 years ago. — number of h +. How To Calculate Halfway Point Titration.
From www.chemistrystudent.com
Finding Ka using a Titration Curve (A2level) ChemistryStudent How To Calculate Halfway Point Titration you can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. Ma is the molarity of the acid, while mb is the molarity of the base. Suppose that a titration is performed and 20.70ml of 0.500mnaoh is. This a fairly straightforward and simple. How To Calculate Halfway Point Titration.
From www.youtube.com
Lecture 7 Titration Half Equivalence Point YouTube How To Calculate Halfway Point Titration Suppose that a titration is performed and 20.70ml of 0.500mnaoh is. you can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. 7.1k views 4 years ago. An explanation and two examples of the halfway point in a titration. how to find. How To Calculate Halfway Point Titration.
From www.chegg.com
Solved The Halfequivalence Point Of A Titration Occurs H... How To Calculate Halfway Point Titration the ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the. the half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. — number of h + ions. Suppose that a titration is performed and. How To Calculate Halfway Point Titration.
From www.numerade.com
SOLVED Calculate the pH at the halfway point and at the equivalence How To Calculate Halfway Point Titration — number of h + ions. 7.1k views 4 years ago. This a fairly straightforward and simple question, however i have found many different. Ma is the molarity of the acid, while mb is the molarity of the base. ma × va = mb × vb. you can use this same approach to calculate the titration curve for. How To Calculate Halfway Point Titration.
From www.youtube.com
Strong acid / strong base titration pH at equivalence point YouTube How To Calculate Halfway Point Titration An explanation and two examples of the halfway point in a titration. how to find the half equivalence point knowing the ph, molarity, titrant added at equivalence point? Va and vb are the volumes of the acid and base, respectively. ma × va = mb × vb. 7.1k views 4 years ago. — number of h + ions.. How To Calculate Halfway Point Titration.
From www.youtube.com
Weak acid / strong base titration pH at equivalence point YouTube How To Calculate Halfway Point Titration ma × va = mb × vb. the half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. Va and vb are the volumes of the acid and base, respectively. you can use this same approach to calculate the titration curve for the titration of a. How To Calculate Halfway Point Titration.
From mehndidesign.zohal.cc
Why Isn T The Ph Value Of Equivalence Point Of A Titration Curve ZOHAL How To Calculate Halfway Point Titration Ma is the molarity of the acid, while mb is the molarity of the base. This a fairly straightforward and simple question, however i have found many different. An explanation and two examples of the halfway point in a titration. you can use this same approach to calculate the titration curve for the titration of a weak base with. How To Calculate Halfway Point Titration.
From staff.buffalostate.edu
AcidBase Reactions How To Calculate Halfway Point Titration Va and vb are the volumes of the acid and base, respectively. This a fairly straightforward and simple question, however i have found many different. you can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. the half equivalence point is the. How To Calculate Halfway Point Titration.
From mungfali.com
Strong Acid And Strong Base Titration How To Calculate Halfway Point Titration ma × va = mb × vb. 7.1k views 4 years ago. This a fairly straightforward and simple question, however i have found many different. Va and vb are the volumes of the acid and base, respectively. how to find the half equivalence point knowing the ph, molarity, titrant added at equivalence point? you can use this. How To Calculate Halfway Point Titration.
From www.numerade.com
SOLVED Consider the titration of 40.0 mL of 0.250 M HF with 0.200 M How To Calculate Halfway Point Titration This a fairly straightforward and simple question, however i have found many different. the ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the. how to find the half equivalence point knowing the ph, molarity, titrant added at equivalence point? An explanation and two examples of. How To Calculate Halfway Point Titration.
From es.howtodoiteasy.com
Se encontró que el "pH" en la mitad del punto de equivalencia en una How To Calculate Halfway Point Titration ma × va = mb × vb. you can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. 7.1k views 4 years ago. how to find the half equivalence point knowing the ph, molarity, titrant added at equivalence point? An explanation. How To Calculate Halfway Point Titration.
From www.chegg.com
Solved Question 1 At which point in the titration curve How To Calculate Halfway Point Titration ma × va = mb × vb. 7.1k views 4 years ago. the half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. This a fairly straightforward and simple question, however i have found many different. how to find the half equivalence point knowing the ph,. How To Calculate Halfway Point Titration.
From fity.club
Acid Base Calculator How To Calculate Halfway Point Titration ma × va = mb × vb. An explanation and two examples of the halfway point in a titration. Va and vb are the volumes of the acid and base, respectively. This a fairly straightforward and simple question, however i have found many different. Ma is the molarity of the acid, while mb is the molarity of the base.. How To Calculate Halfway Point Titration.
From theedge.com.hk
Chemistry How To Titration The Edge How To Calculate Halfway Point Titration the half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. you can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. — number of h + ions. This a fairly. How To Calculate Halfway Point Titration.
From www.youtube.com
Titration Curves, Equivalence Point YouTube How To Calculate Halfway Point Titration you can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. This a fairly straightforward and simple question, however i have found many different. — number of h + ions. Ma is the molarity of the acid, while mb is the molarity of. How To Calculate Halfway Point Titration.
From socratic.org
How many moles of HX have been added at the equivalence point? Socratic How To Calculate Halfway Point Titration ma × va = mb × vb. the ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the. you can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is.. How To Calculate Halfway Point Titration.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 How To Calculate Halfway Point Titration the ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the. Ma is the molarity of the acid, while mb is the molarity of the base. you can use this same approach to calculate the titration curve for the titration of a weak base with a. How To Calculate Halfway Point Titration.
From knowledge-builders.org
What Is The Ph At The Second Equivalence Point Of The Titration How To Calculate Halfway Point Titration This a fairly straightforward and simple question, however i have found many different. how to find the half equivalence point knowing the ph, molarity, titrant added at equivalence point? Suppose that a titration is performed and 20.70ml of 0.500mnaoh is. — number of h + ions. 7.1k views 4 years ago. the ph at the midpoint, the point. How To Calculate Halfway Point Titration.
From fity.club
Titration Calculations How To Calculate Halfway Point Titration An explanation and two examples of the halfway point in a titration. Suppose that a titration is performed and 20.70ml of 0.500mnaoh is. This a fairly straightforward and simple question, however i have found many different. Va and vb are the volumes of the acid and base, respectively. the half equivalence point is the stage of the titration at. How To Calculate Halfway Point Titration.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation, free How To Calculate Halfway Point Titration 7.1k views 4 years ago. — number of h + ions. you can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. Ma is the molarity of the acid, while mb is the molarity of the base. the half equivalence point is. How To Calculate Halfway Point Titration.
From www.numerade.com
SOLVED For the titration curve below, where an unknown weak acid was How To Calculate Halfway Point Titration the ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the. This a fairly straightforward and simple question, however i have found many different. how to find the half equivalence point knowing the ph, molarity, titrant added at equivalence point? 7.1k views 4 years ago. Ma. How To Calculate Halfway Point Titration.
From mungfali.com
Half Equivalence Point On Titration Curve How To Calculate Halfway Point Titration the ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the. you can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. Suppose that a titration is performed and 20.70ml. How To Calculate Halfway Point Titration.
From perso.numericable.fr
At the point halfway to equivalence pH=pKa or pOH=pKb and pH+pOH = 14 How To Calculate Halfway Point Titration Suppose that a titration is performed and 20.70ml of 0.500mnaoh is. ma × va = mb × vb. Ma is the molarity of the acid, while mb is the molarity of the base. the half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. This a fairly. How To Calculate Halfway Point Titration.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki How To Calculate Halfway Point Titration the half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. ma × va = mb × vb. An explanation and two examples of the halfway point in a titration. — number of h + ions. Va and vb are the volumes of the acid and base,. How To Calculate Halfway Point Titration.
From www.youtube.com
Halfway Point in Titration YouTube How To Calculate Halfway Point Titration — number of h + ions. ma × va = mb × vb. 7.1k views 4 years ago. how to find the half equivalence point knowing the ph, molarity, titrant added at equivalence point? Ma is the molarity of the acid, while mb is the molarity of the base. the ph at the midpoint, the point halfway. How To Calculate Halfway Point Titration.
From www.numerade.com
SOLVEDA 20.0 mL sample of 0.125 M HNO3 is titrated with 0.150 M NaOH How To Calculate Halfway Point Titration Ma is the molarity of the acid, while mb is the molarity of the base. Suppose that a titration is performed and 20.70ml of 0.500mnaoh is. how to find the half equivalence point knowing the ph, molarity, titrant added at equivalence point? ma × va = mb × vb. the half equivalence point is the stage of. How To Calculate Halfway Point Titration.
From www.vrogue.co
Acid Base Titrations · Chemistry Titration Curves Equivalence Point How To Calculate Halfway Point Titration you can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. Va and vb are the volumes of the acid and base, respectively. An explanation and two examples of the halfway point in a titration. Ma is the molarity of the acid, while. How To Calculate Halfway Point Titration.