Redox Titration With Kmno4 . the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant for determining the. titrate with kmno4 at 60~90 oc with stirring. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an. Iron (ii) with potassium permanganate. in this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. 30 s) pink color as the end point. here are a few examples of redox titrations, along with their corresponding chemical equations:
from www.chegg.com
in this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. 30 s) pink color as the end point. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an. here are a few examples of redox titrations, along with their corresponding chemical equations: Iron (ii) with potassium permanganate. because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant for determining the. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. titrate with kmno4 at 60~90 oc with stirring.
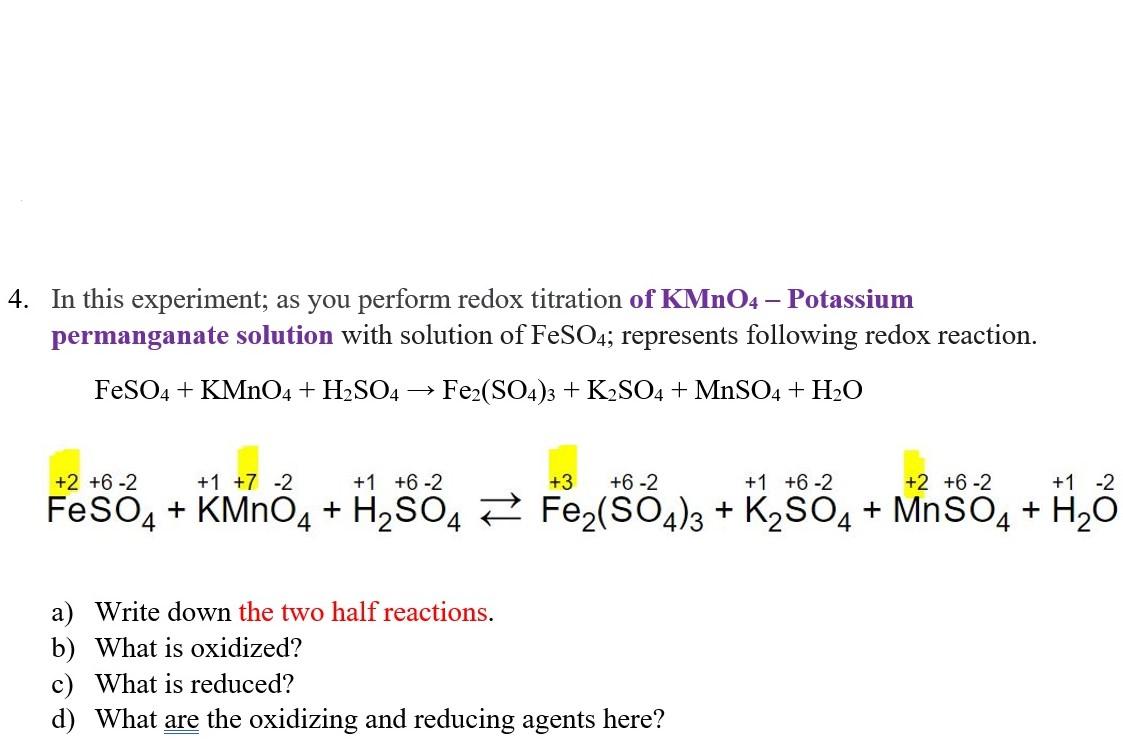
Solved 4. In this experiment; as you perform redox titration
Redox Titration With Kmno4 because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant for determining the. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an. because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant for determining the. titrate with kmno4 at 60~90 oc with stirring. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. Iron (ii) with potassium permanganate. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. 30 s) pink color as the end point. in this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the. here are a few examples of redox titrations, along with their corresponding chemical equations:
From www.chegg.com
Solved Acidic solution In acidic solution, the bromate ion Redox Titration With Kmno4 titrate with kmno4 at 60~90 oc with stirring. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Iron (ii) with potassium permanganate. in this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the. 30 s) pink. Redox Titration With Kmno4.
From www.numerade.com
SOLVED Redox Titration Potassium permanganate, KMnO4, is a strong Redox Titration With Kmno4 in this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the. 30 s) pink color as the end point. here are a few examples of redox titrations, along with their corresponding chemical equations: the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4). Redox Titration With Kmno4.
From www.youtube.com
Titration of KMnO4 vs Ferrous ammonium sulphate Redox titrationHow to Redox Titration With Kmno4 the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. in this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the. titrate with kmno4 at 60~90 oc with stirring. 30 s) pink color as the end point. Iron. Redox Titration With Kmno4.
From www.slideserve.com
PPT REDOX TITRATIONS PowerPoint Presentation, free download ID2670469 Redox Titration With Kmno4 in this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as. Redox Titration With Kmno4.
From www.youtube.com
KMnO4 Redox Titration With FeSO4.7H20 YouTube Redox Titration With Kmno4 the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. here are a few examples of redox titrations, along with their corresponding chemical equations: because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant for determining the.. Redox Titration With Kmno4.
From www.numerade.com
SOLVED Text LESSON Redox Titration Lab Titration Redox Titration Redox Titration With Kmno4 30 s) pink color as the end point. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant for determining the. Iron (ii) with potassium permanganate. . Redox Titration With Kmno4.
From slideplayer.com
Titration Colour Changes ppt download Redox Titration With Kmno4 titrate with kmno4 at 60~90 oc with stirring. here are a few examples of redox titrations, along with their corresponding chemical equations: the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. because this reaction is rapid and goes to completion, potassium permanganate (kmno. Redox Titration With Kmno4.
From www.studypool.com
SOLUTION Redox titration Studypool Redox Titration With Kmno4 In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. in this titration process, the oxalic acid acts as the reducing agent. Redox Titration With Kmno4.
From www.numerade.com
SOLVEDRedox Titrationi Acidic Medium Puch Slider Upto Add Volume of Redox Titration With Kmno4 in this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an. here are a few examples of redox titrations, along with their corresponding chemical equations: . Redox Titration With Kmno4.
From slideplayer.com
Electrochemistry Chemical reactions and Electricity ppt download Redox Titration With Kmno4 the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. titrate with kmno4 at 60~90 oc with stirring. In this experiment you will use. Redox Titration With Kmno4.
From slideplayer.com
Unit 12 (Chp 20) Electrochemistry (E, ∆G, K) ppt download Redox Titration With Kmno4 30 s) pink color as the end point. here are a few examples of redox titrations, along with their corresponding chemical equations: titrate with kmno4 at 60~90 oc with stirring. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an. because this reaction. Redox Titration With Kmno4.
From www.youtube.com
Redox Titration between MnO4 and Fe2+ YouTube Redox Titration With Kmno4 In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an. in this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the. Iron (ii) with potassium permanganate. because this reaction is rapid and goes to completion, potassium. Redox Titration With Kmno4.
From www.numerade.com
SOLVED 0.04M of KMnO4 solution was titrated with 1.00 ml of (13.4 g Redox Titration With Kmno4 because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant for determining the. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an. 30 s) pink color as the end point. in this titration process,. Redox Titration With Kmno4.
From chemistrypubs.com
Titration of Oxalic Acid with KMnO4 Chemistrupubs Redox Titration With Kmno4 titrate with kmno4 at 60~90 oc with stirring. in this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. 30 s) pink color as the end point.. Redox Titration With Kmno4.
From exyylkanq.blob.core.windows.net
Titration General Chemistry at Bruce Fagan blog Redox Titration With Kmno4 here are a few examples of redox titrations, along with their corresponding chemical equations: Iron (ii) with potassium permanganate. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an. in this titration process, the oxalic acid acts as the reducing agent while potassium permanganate. Redox Titration With Kmno4.
From www.flinnsci.com
Analysis of Hydrogen Peroxide A Redox Titration—ChemTopic™ Lab Redox Titration With Kmno4 the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. Iron (ii) with potassium permanganate. titrate with kmno4 at 60~90 oc with stirring. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an.. Redox Titration With Kmno4.
From www.coursehero.com
[Solved] . Redox titrations are used to determine the amounts of Redox Titration With Kmno4 the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. in this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the. here are a few examples of redox titrations, along with their corresponding chemical equations: 30 s). Redox Titration With Kmno4.
From www.chegg.com
Solved 4. In this experiment; as you perform redox titration Redox Titration With Kmno4 the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. in this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the. Iron (ii) with potassium permanganate. here are a few examples of redox titrations, along with their corresponding. Redox Titration With Kmno4.
From fyonleavi.blob.core.windows.net
Kmno4 Titration Lab at Sherron Horn blog Redox Titration With Kmno4 30 s) pink color as the end point. Iron (ii) with potassium permanganate. here are a few examples of redox titrations, along with their corresponding chemical equations: In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an. in this titration process, the oxalic acid. Redox Titration With Kmno4.
From www.slideserve.com
PPT REDOX TITRATION PowerPoint Presentation, free download ID4545121 Redox Titration With Kmno4 30 s) pink color as the end point. in this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the. here are a few examples of redox titrations, along with their corresponding chemical equations: In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the. Redox Titration With Kmno4.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记8.2.1 Redox Titration翰林国际教育 Redox Titration With Kmno4 the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. 30 s) pink color as the end point. because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant for determining the. Iron (ii) with potassium permanganate. In. Redox Titration With Kmno4.
From exybsrswn.blob.core.windows.net
Redox Titration Kmno4 at Amanda Young blog Redox Titration With Kmno4 because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant for determining the. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2. Redox Titration With Kmno4.
From www.slideserve.com
PPT Chemical Reactions in Aqueous Solutions PowerPoint Presentation Redox Titration With Kmno4 in this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the. because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant for determining the. titrate with kmno4 at 60~90 oc with stirring. In this experiment you will use a standard. Redox Titration With Kmno4.
From www.slideserve.com
PPT Ch 16 Redox Titrations PowerPoint Presentation, free download Redox Titration With Kmno4 here are a few examples of redox titrations, along with their corresponding chemical equations: in this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the. 30 s) pink color as the end point. Iron (ii) with potassium permanganate. the titration of potassium permanganate (kmno 4) against oxalic acid (c. Redox Titration With Kmno4.
From www.slideserve.com
PPT REDOX TITRATION PowerPoint Presentation, free download ID431911 Redox Titration With Kmno4 30 s) pink color as the end point. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an. titrate with kmno4 at. Redox Titration With Kmno4.
From www.youtube.com
Titration KMnO4 Vs Mohr's Salt Full Experiment + Calculation YouTube Redox Titration With Kmno4 here are a few examples of redox titrations, along with their corresponding chemical equations: because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant for determining the. 30 s) pink color as the end point. titrate with kmno4 at 60~90 oc with stirring. the titration of potassium. Redox Titration With Kmno4.
From www.pdffiller.com
Fillable Online C14 Redox Titration with KMnO4 Fax Email Print pdfFiller Redox Titration With Kmno4 the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. in this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of. Redox Titration With Kmno4.
From www.youtube.com
Redox Titration II KMnO4 vs Oxalic acid II Self indicator YouTube Redox Titration With Kmno4 titrate with kmno4 at 60~90 oc with stirring. here are a few examples of redox titrations, along with their corresponding chemical equations: because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant for determining the. In this experiment you will use a standard solution of potassium permanganate (kmno. Redox Titration With Kmno4.
From www.numerade.com
SOLVED MnO4 + H2O2 > Mn2+ + O2 The above reaction was used for a Redox Titration With Kmno4 Iron (ii) with potassium permanganate. in this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the. because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant for determining the. the redox titration of permanganate ions is commonly used to determine. Redox Titration With Kmno4.
From www.youtube.com
Standardization of KMnO4 Redox Titration KMnO4 Vs Fe2+ Mohrs salt Redox Titration With Kmno4 Iron (ii) with potassium permanganate. because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant for determining the. the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. in this titration process, the oxalic acid acts as. Redox Titration With Kmno4.
From www.youtube.com
Determination of Concentration of KMnO4 Soution using Ferrous Ammonium Redox Titration With Kmno4 the redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant for determining the. here are a few examples of redox titrations, along with their corresponding chemical equations:. Redox Titration With Kmno4.
From fyonleavi.blob.core.windows.net
Kmno4 Titration Lab at Sherron Horn blog Redox Titration With Kmno4 because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant for determining the. 30 s) pink color as the end point. here are a few examples of redox titrations, along with their corresponding chemical equations: the redox titration of permanganate ions is commonly used to determine the iron. Redox Titration With Kmno4.
From slideplayer.com
Changes in matter involve the rearrangement and/or of Redox Titration With Kmno4 Iron (ii) with potassium permanganate. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an. 30 s) pink color as the end point. because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant for determining the.. Redox Titration With Kmno4.
From www.thesciencehive.co.uk
Redox and Electrode Potentials* — the science sauce Redox Titration With Kmno4 30 s) pink color as the end point. Iron (ii) with potassium permanganate. because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant for determining the. titrate with kmno4 at 60~90 oc with stirring. here are a few examples of redox titrations, along with their corresponding chemical equations:. Redox Titration With Kmno4.
From exybsrswn.blob.core.windows.net
Redox Titration Kmno4 at Amanda Young blog Redox Titration With Kmno4 Iron (ii) with potassium permanganate. in this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. In this experiment you will use a standard solution of potassium permanganate. Redox Titration With Kmno4.