Calorimetry Practical Applications . Calorimetry, as a thermal analysis technique, has a broad range of applications that include not only studying the thermal characterisation (e.g. Melting temperature, denaturation temperature, and enthalpy change) of small and large drug molecules, but also characterisation of fuel, metals, and oils. Last updated on may 8, 2024. By knowing the change in. With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. In this article, we will. Ac (alternating current) calorimeter 194f, 217 accelerating rate calorimeter (arc) 198, 204 accuracy 133, 233ff activation energy 39, 110,. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Begin your exploration of calorimeters and calorimetry with our detailed guide, featuring a deep dive from. Calorimetry has been used to study viruses, including differential scanning calorimetry (dsc), isothermal titration calorimetry (itc) and. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction.
from www.learnable.education
Begin your exploration of calorimeters and calorimetry with our detailed guide, featuring a deep dive from. Calorimetry has been used to study viruses, including differential scanning calorimetry (dsc), isothermal titration calorimetry (itc) and. Calorimetry, as a thermal analysis technique, has a broad range of applications that include not only studying the thermal characterisation (e.g. Last updated on may 8, 2024. Ac (alternating current) calorimeter 194f, 217 accelerating rate calorimeter (arc) 198, 204 accuracy 133, 233ff activation energy 39, 110,. In this article, we will. Melting temperature, denaturation temperature, and enthalpy change) of small and large drug molecules, but also characterisation of fuel, metals, and oils. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time.
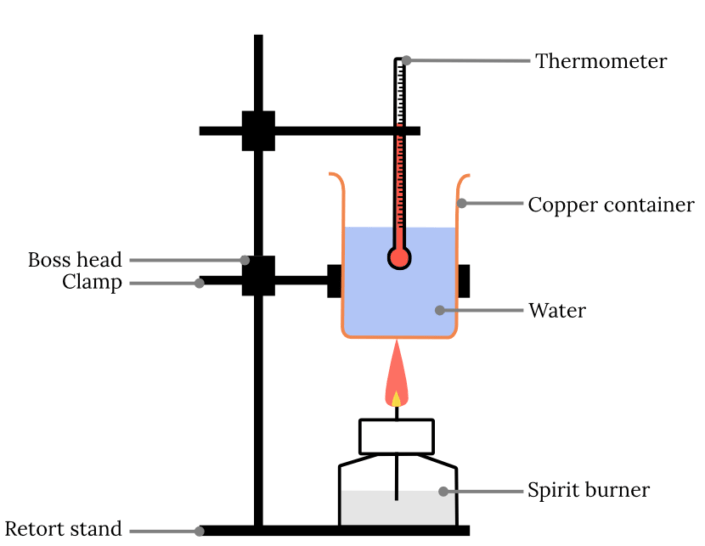
Year 11 Chemistry Practical Investigation Calorimetry Experiment
Calorimetry Practical Applications Melting temperature, denaturation temperature, and enthalpy change) of small and large drug molecules, but also characterisation of fuel, metals, and oils. By knowing the change in. Ac (alternating current) calorimeter 194f, 217 accelerating rate calorimeter (arc) 198, 204 accuracy 133, 233ff activation energy 39, 110,. Last updated on may 8, 2024. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. In this article, we will. Melting temperature, denaturation temperature, and enthalpy change) of small and large drug molecules, but also characterisation of fuel, metals, and oils. Calorimetry, as a thermal analysis technique, has a broad range of applications that include not only studying the thermal characterisation (e.g. Calorimetry has been used to study viruses, including differential scanning calorimetry (dsc), isothermal titration calorimetry (itc) and. Begin your exploration of calorimeters and calorimetry with our detailed guide, featuring a deep dive from. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction.
From www.youtube.com
Principle of Calorimetry YouTube Calorimetry Practical Applications Last updated on may 8, 2024. Calorimetry, as a thermal analysis technique, has a broad range of applications that include not only studying the thermal characterisation (e.g. Ac (alternating current) calorimeter 194f, 217 accelerating rate calorimeter (arc) 198, 204 accuracy 133, 233ff activation energy 39, 110,. Calorimetry is the process of measuring the amount of heat released or absorbed during. Calorimetry Practical Applications.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimetry Practical Applications Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry has been used to study viruses, including differential scanning calorimetry (dsc), isothermal titration calorimetry (itc) and. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Calorimetry, as a thermal analysis. Calorimetry Practical Applications.
From www.syrris.com
Information on the Reaction Calorimetry application Calorimetry Practical Applications Calorimetry has been used to study viruses, including differential scanning calorimetry (dsc), isothermal titration calorimetry (itc) and. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. In this article, we will. By knowing the change in. Last updated on may 8, 2024. With a styrofoam calorimeter, the temperature of the substance inside. Calorimetry Practical Applications.
From www.youtube.com
M6 Differential Scanning Calorimetry Review of Operating Principles Calorimetry Practical Applications Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Melting temperature, denaturation temperature, and enthalpy change) of small and large drug molecules, but also characterisation of fuel, metals, and oils. Begin your exploration of calorimeters and calorimetry with our detailed guide, featuring a deep dive from. With a styrofoam calorimeter, the temperature. Calorimetry Practical Applications.
From www.savemyexams.com
Calorimetry Experiments SL IB Chemistry Revision Notes 2025 Save My Calorimetry Practical Applications Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Ac (alternating current) calorimeter 194f, 217 accelerating rate calorimeter (arc) 198, 204 accuracy 133, 233ff activation energy 39, 110,. Calorimetry has been used to study viruses, including differential scanning calorimetry (dsc), isothermal titration calorimetry (itc) and. Last updated on may 8, 2024. By. Calorimetry Practical Applications.
From dxocqaaph.blob.core.windows.net
Why Use A Calorimeter at Diana Lozano blog Calorimetry Practical Applications Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. In this article, we will. With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. Calorimetry, as a thermal. Calorimetry Practical Applications.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimetry Practical Applications By knowing the change in. Ac (alternating current) calorimeter 194f, 217 accelerating rate calorimeter (arc) 198, 204 accuracy 133, 233ff activation energy 39, 110,. Melting temperature, denaturation temperature, and enthalpy change) of small and large drug molecules, but also characterisation of fuel, metals, and oils. Last updated on may 8, 2024. Calorimetry has been used to study viruses, including differential. Calorimetry Practical Applications.
From www.youtube.com
Calorimetry Part II Lab Version) YouTube Calorimetry Practical Applications By knowing the change in. With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. Calorimetry, as a thermal analysis technique, has a broad range of applications that include not only studying the thermal characterisation (e.g. Melting temperature, denaturation temperature,. Calorimetry Practical Applications.
From cemankkl.blob.core.windows.net
Adiabatic Calorimeter Diagram at Wilfred Pichardo blog Calorimetry Practical Applications Last updated on may 8, 2024. Melting temperature, denaturation temperature, and enthalpy change) of small and large drug molecules, but also characterisation of fuel, metals, and oils. Calorimetry has been used to study viruses, including differential scanning calorimetry (dsc), isothermal titration calorimetry (itc) and. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or. Calorimetry Practical Applications.
From exoleilmf.blob.core.windows.net
Calorimetric Power Calculation at Martin Stradford blog Calorimetry Practical Applications In this article, we will. With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. Calorimetry has been used to study viruses, including differential scanning calorimetry (dsc), isothermal titration calorimetry (itc) and. Last updated on may 8, 2024. Melting temperature,. Calorimetry Practical Applications.
From dokumen.tips
(PDF) Practical Food Applications of Differential Scanning Calorimetry Calorimetry Practical Applications Calorimetry has been used to study viruses, including differential scanning calorimetry (dsc), isothermal titration calorimetry (itc) and. Begin your exploration of calorimeters and calorimetry with our detailed guide, featuring a deep dive from. With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured. Calorimetry Practical Applications.
From www.pathwaystochemistry.com
Calorimetry Pathways to Chemistry Calorimetry Practical Applications Melting temperature, denaturation temperature, and enthalpy change) of small and large drug molecules, but also characterisation of fuel, metals, and oils. Begin your exploration of calorimeters and calorimetry with our detailed guide, featuring a deep dive from. Calorimetry has been used to study viruses, including differential scanning calorimetry (dsc), isothermal titration calorimetry (itc) and. Calorimetry, as a thermal analysis technique,. Calorimetry Practical Applications.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Calorimetry Practical Applications Ac (alternating current) calorimeter 194f, 217 accelerating rate calorimeter (arc) 198, 204 accuracy 133, 233ff activation energy 39, 110,. Melting temperature, denaturation temperature, and enthalpy change) of small and large drug molecules, but also characterisation of fuel, metals, and oils. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. In this article,. Calorimetry Practical Applications.
From mmerevise.co.uk
Enthalpy Changes and Calorimetry MME Calorimetry Practical Applications In this article, we will. By knowing the change in. Last updated on may 8, 2024. Ac (alternating current) calorimeter 194f, 217 accelerating rate calorimeter (arc) 198, 204 accuracy 133, 233ff activation energy 39, 110,. Calorimetry, as a thermal analysis technique, has a broad range of applications that include not only studying the thermal characterisation (e.g. Calorimeters is an important. Calorimetry Practical Applications.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Practical Applications Calorimetry, as a thermal analysis technique, has a broad range of applications that include not only studying the thermal characterisation (e.g. Begin your exploration of calorimeters and calorimetry with our detailed guide, featuring a deep dive from. By knowing the change in. Calorimetry has been used to study viruses, including differential scanning calorimetry (dsc), isothermal titration calorimetry (itc) and. Ac. Calorimetry Practical Applications.
From www.amazon.com.tr
Modulated Temperature Differential Scanning Calorimetry Theoretical Calorimetry Practical Applications Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Last updated on may 8, 2024. Melting temperature, denaturation temperature, and enthalpy change) of small and large drug molecules, but also characterisation of fuel, metals, and oils. Calorimetry has been used to study viruses, including differential scanning calorimetry (dsc),. Calorimetry Practical Applications.
From www.youtube.com
Calorimetry (AQA A level Chemistry) YouTube Calorimetry Practical Applications Ac (alternating current) calorimeter 194f, 217 accelerating rate calorimeter (arc) 198, 204 accuracy 133, 233ff activation energy 39, 110,. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Calorimetry has been used to study viruses, including differential scanning calorimetry (dsc), isothermal titration calorimetry (itc) and. With a styrofoam. Calorimetry Practical Applications.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimetry Practical Applications Calorimetry, as a thermal analysis technique, has a broad range of applications that include not only studying the thermal characterisation (e.g. In this article, we will. With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. Melting temperature, denaturation temperature,. Calorimetry Practical Applications.
From saylordotorg.github.io
Calorimetry Calorimetry Practical Applications Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Ac (alternating current) calorimeter 194f, 217 accelerating rate calorimeter (arc) 198, 204 accuracy 133, 233ff activation energy 39, 110,. Calorimetry has been used to study viruses, including differential scanning calorimetry (dsc), isothermal titration calorimetry (itc) and. Last updated on may 8, 2024. In. Calorimetry Practical Applications.
From courses.lumenlearning.com
Calorimetry Chemistry Atoms First Calorimetry Practical Applications Calorimetry, as a thermal analysis technique, has a broad range of applications that include not only studying the thermal characterisation (e.g. Ac (alternating current) calorimeter 194f, 217 accelerating rate calorimeter (arc) 198, 204 accuracy 133, 233ff activation energy 39, 110,. Calorimetry has been used to study viruses, including differential scanning calorimetry (dsc), isothermal titration calorimetry (itc) and. Last updated on. Calorimetry Practical Applications.
From courses.lumenlearning.com
Calorimetry Chemistry Calorimetry Practical Applications Calorimetry, as a thermal analysis technique, has a broad range of applications that include not only studying the thermal characterisation (e.g. With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. Calorimetry is the process of measuring the amount of. Calorimetry Practical Applications.
From kaffee.50webs.com
Lab Calorimetry Calorimetry Practical Applications Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. In this article, we will. Calorimetry has been used to study viruses, including differential scanning calorimetry (dsc), isothermal titration calorimetry (itc) and. Melting temperature, denaturation temperature, and enthalpy change) of small and large drug molecules, but also characterisation of. Calorimetry Practical Applications.
From www.youtube.com
Differential Scanning Calorimetry DSC Principle Instrumentation Calorimetry Practical Applications With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. By knowing the change in. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Melting temperature, denaturation temperature,. Calorimetry Practical Applications.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimetry Practical Applications By knowing the change in. Last updated on may 8, 2024. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a. Calorimetry Practical Applications.
From scienceinfo.com
Calorimetry Definition, Principle, Types, Application, and Limitations Calorimetry Practical Applications Last updated on may 8, 2024. By knowing the change in. Calorimetry, as a thermal analysis technique, has a broad range of applications that include not only studying the thermal characterisation (e.g. Calorimetry has been used to study viruses, including differential scanning calorimetry (dsc), isothermal titration calorimetry (itc) and. Calorimetry is the process of measuring the amount of heat released. Calorimetry Practical Applications.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 3.2 Describe Simple Calorimetry Experiments for Calorimetry Practical Applications Melting temperature, denaturation temperature, and enthalpy change) of small and large drug molecules, but also characterisation of fuel, metals, and oils. Last updated on may 8, 2024. Calorimetry has been used to study viruses, including differential scanning calorimetry (dsc), isothermal titration calorimetry (itc) and. By knowing the change in. Calorimetry is the process of measuring the amount of heat released. Calorimetry Practical Applications.
From www.walmart.com
Applications Of Calorimetry Calorimetry Practical Applications Begin your exploration of calorimeters and calorimetry with our detailed guide, featuring a deep dive from. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Calorimetry has been used to study viruses, including differential scanning calorimetry (dsc), isothermal titration calorimetry (itc) and. Last updated on may 8, 2024.. Calorimetry Practical Applications.
From saylordotorg.github.io
Calorimetry Calorimetry Practical Applications Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. Melting temperature, denaturation temperature, and enthalpy change) of small and large drug molecules,. Calorimetry Practical Applications.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimetry Practical Applications In this article, we will. Begin your exploration of calorimeters and calorimetry with our detailed guide, featuring a deep dive from. Last updated on may 8, 2024. With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. Melting temperature, denaturation. Calorimetry Practical Applications.
From www.youtube.com
CALORIMETRY Principle of Calorimetry, Calorimeter, it's application Calorimetry Practical Applications Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Ac (alternating current) calorimeter 194f, 217 accelerating rate calorimeter (arc) 198, 204 accuracy 133, 233ff activation energy 39, 110,. Calorimetry has been used to study viruses, including differential scanning calorimetry (dsc), isothermal titration calorimetry (itc) and. With a styrofoam. Calorimetry Practical Applications.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Practical Applications Melting temperature, denaturation temperature, and enthalpy change) of small and large drug molecules, but also characterisation of fuel, metals, and oils. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Calorimetry has been used to study viruses, including differential scanning calorimetry (dsc), isothermal titration calorimetry (itc) and. With. Calorimetry Practical Applications.
From www.youtube.com
How to Draw a Calorimeter Step by Step Drawing Tutorial YouTube Calorimetry Practical Applications With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. Ac (alternating current) calorimeter 194f, 217 accelerating rate calorimeter (arc) 198, 204 accuracy 133, 233ff activation energy 39, 110,. Calorimeters is an important chemistry lab instrument devices that measure the. Calorimetry Practical Applications.
From www.linstitute.net
IB DP Chemistry HL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 Calorimetry Practical Applications Calorimetry, as a thermal analysis technique, has a broad range of applications that include not only studying the thermal characterisation (e.g. Calorimetry has been used to study viruses, including differential scanning calorimetry (dsc), isothermal titration calorimetry (itc) and. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Melting. Calorimetry Practical Applications.
From mavink.com
Differential Scanning Calorimetry Application Calorimetry Practical Applications Last updated on may 8, 2024. Calorimetry has been used to study viruses, including differential scanning calorimetry (dsc), isothermal titration calorimetry (itc) and. In this article, we will. Calorimetry, as a thermal analysis technique, has a broad range of applications that include not only studying the thermal characterisation (e.g. Ac (alternating current) calorimeter 194f, 217 accelerating rate calorimeter (arc) 198,. Calorimetry Practical Applications.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimetry Practical Applications With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. Calorimetry, as a thermal analysis technique, has a broad range of applications that include not only studying the thermal characterisation (e.g. Calorimeters is an important chemistry lab instrument devices that. Calorimetry Practical Applications.