Indicator Used In Permanganometric Titration . Oxidation of fe 2+ by permanganate is one of the most popular. Generally for the titration process, external indicators are used to predict the completion of a titration process, but in the case of potassium permanganate titration, kmno 4 acts as a self. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. In close proximity to the endpoint, the action of the indicator is. There are two basic types of acid base titrations, indicator and potentiometric. Best results can be obtained when permanganate is used for. In an indicator based titration you add another. Between a reference electrode and an indicator (redox) electrode at. Permanganate requires low ph, or presence of strong acid. This is a procedure which depends upon measurement of the e.m.f. Permanganometric titration of iron (ii) general remarks.
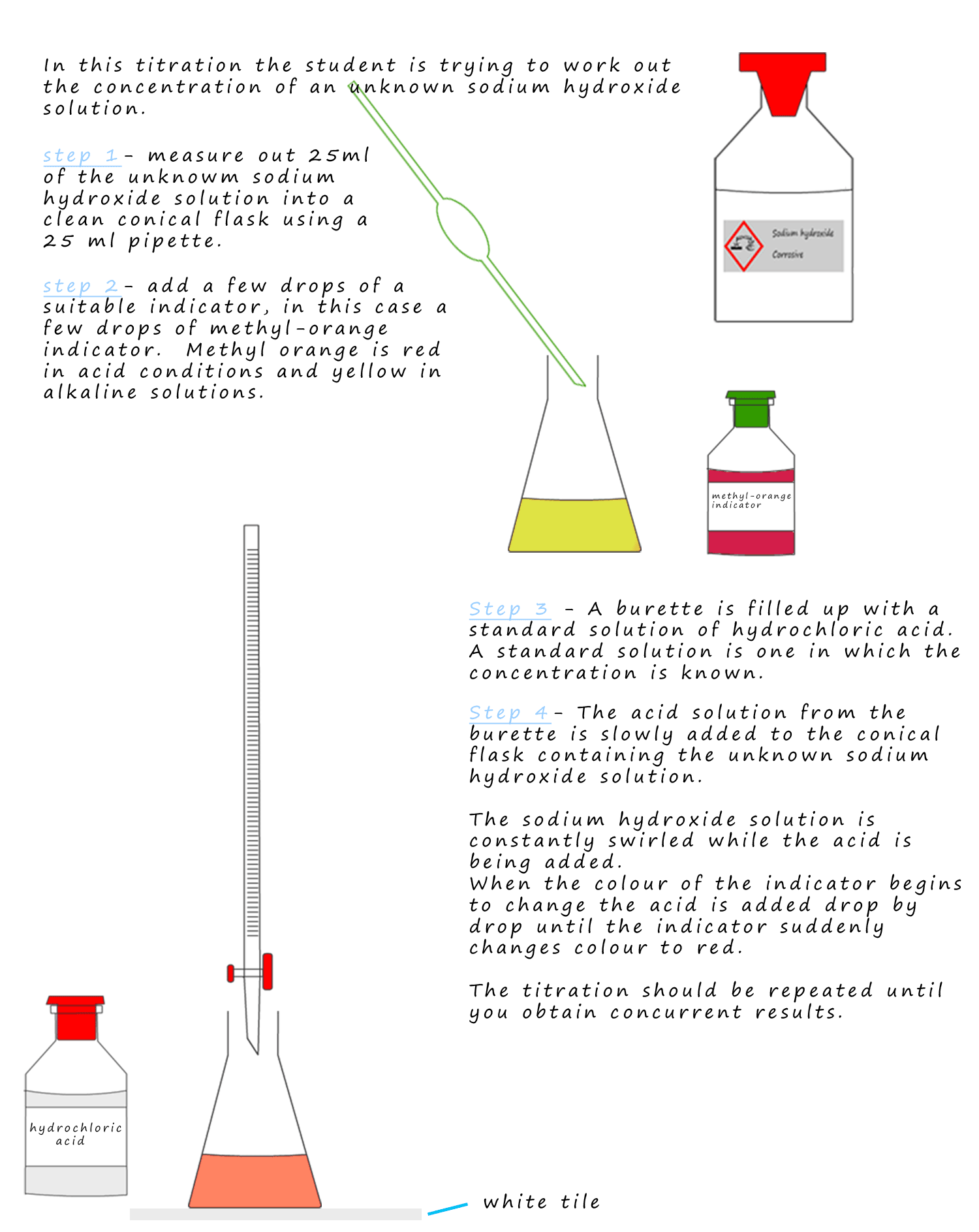
from www.science-revision.co.uk
There are two basic types of acid base titrations, indicator and potentiometric. Permanganate requires low ph, or presence of strong acid. This is a procedure which depends upon measurement of the e.m.f. In close proximity to the endpoint, the action of the indicator is. In an indicator based titration you add another. Between a reference electrode and an indicator (redox) electrode at. Generally for the titration process, external indicators are used to predict the completion of a titration process, but in the case of potassium permanganate titration, kmno 4 acts as a self. Oxidation of fe 2+ by permanganate is one of the most popular. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Best results can be obtained when permanganate is used for.
Titrations
Indicator Used In Permanganometric Titration In close proximity to the endpoint, the action of the indicator is. In close proximity to the endpoint, the action of the indicator is. There are two basic types of acid base titrations, indicator and potentiometric. Generally for the titration process, external indicators are used to predict the completion of a titration process, but in the case of potassium permanganate titration, kmno 4 acts as a self. Best results can be obtained when permanganate is used for. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. In an indicator based titration you add another. Permanganate requires low ph, or presence of strong acid. Permanganometric titration of iron (ii) general remarks. Between a reference electrode and an indicator (redox) electrode at. This is a procedure which depends upon measurement of the e.m.f. Oxidation of fe 2+ by permanganate is one of the most popular.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Indicator Used In Permanganometric Titration Permanganometric titration of iron (ii) general remarks. In an indicator based titration you add another. Oxidation of fe 2+ by permanganate is one of the most popular. This is a procedure which depends upon measurement of the e.m.f. Permanganate requires low ph, or presence of strong acid. There are two basic types of acid base titrations, indicator and potentiometric. In. Indicator Used In Permanganometric Titration.
From pubs.acs.org
Permanganometric Titration for the Quantification of Purified Bis(2,4,4 Indicator Used In Permanganometric Titration Generally for the titration process, external indicators are used to predict the completion of a titration process, but in the case of potassium permanganate titration, kmno 4 acts as a self. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. There are two basic types of acid. Indicator Used In Permanganometric Titration.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Indicator Used In Permanganometric Titration Permanganate requires low ph, or presence of strong acid. Generally for the titration process, external indicators are used to predict the completion of a titration process, but in the case of potassium permanganate titration, kmno 4 acts as a self. In an indicator based titration you add another. Permanganometric titration of iron (ii) general remarks. There are two basic types. Indicator Used In Permanganometric Titration.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Indicator Used In Permanganometric Titration The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Permanganate requires low ph, or presence of strong acid. Oxidation of fe 2+ by permanganate is one of the most popular. There are two basic types of acid base titrations, indicator and potentiometric. In close proximity to the. Indicator Used In Permanganometric Titration.
From www.slideserve.com
PPT Volumetric ANALYSIS/TITRATION PowerPoint Presentation, free Indicator Used In Permanganometric Titration Permanganate requires low ph, or presence of strong acid. There are two basic types of acid base titrations, indicator and potentiometric. Between a reference electrode and an indicator (redox) electrode at. Generally for the titration process, external indicators are used to predict the completion of a titration process, but in the case of potassium permanganate titration, kmno 4 acts as. Indicator Used In Permanganometric Titration.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID5572517 Indicator Used In Permanganometric Titration There are two basic types of acid base titrations, indicator and potentiometric. Permanganometric titration of iron (ii) general remarks. Best results can be obtained when permanganate is used for. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Generally for the titration process, external indicators are used. Indicator Used In Permanganometric Titration.
From www.youtube.com
Viva voce Questions on Permanganometric Titration (KMnO4 vs Oxalic acid Indicator Used In Permanganometric Titration In close proximity to the endpoint, the action of the indicator is. Between a reference electrode and an indicator (redox) electrode at. Oxidation of fe 2+ by permanganate is one of the most popular. This is a procedure which depends upon measurement of the e.m.f. Permanganate requires low ph, or presence of strong acid. The titration of potassium permanganate (kmno. Indicator Used In Permanganometric Titration.
From hxevhdmch.blob.core.windows.net
What Is A Titration Indicator at Kelly Ayala blog Indicator Used In Permanganometric Titration Best results can be obtained when permanganate is used for. Between a reference electrode and an indicator (redox) electrode at. Permanganometric titration of iron (ii) general remarks. There are two basic types of acid base titrations, indicator and potentiometric. Generally for the titration process, external indicators are used to predict the completion of a titration process, but in the case. Indicator Used In Permanganometric Titration.
From www.studypool.com
SOLUTION Indicators used in titration Studypool Indicator Used In Permanganometric Titration In close proximity to the endpoint, the action of the indicator is. This is a procedure which depends upon measurement of the e.m.f. Permanganometric titration of iron (ii) general remarks. There are two basic types of acid base titrations, indicator and potentiometric. Permanganate requires low ph, or presence of strong acid. Best results can be obtained when permanganate is used. Indicator Used In Permanganometric Titration.
From www.youtube.com
IGCSE Chemistry Titration and indicators method question YouTube Indicator Used In Permanganometric Titration Permanganate requires low ph, or presence of strong acid. There are two basic types of acid base titrations, indicator and potentiometric. In close proximity to the endpoint, the action of the indicator is. This is a procedure which depends upon measurement of the e.m.f. Generally for the titration process, external indicators are used to predict the completion of a titration. Indicator Used In Permanganometric Titration.
From www.youtube.com
Permanganometry ( Redox Titration) 17 Titration YouTube Indicator Used In Permanganometric Titration Best results can be obtained when permanganate is used for. There are two basic types of acid base titrations, indicator and potentiometric. Between a reference electrode and an indicator (redox) electrode at. Permanganate requires low ph, or presence of strong acid. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example. Indicator Used In Permanganometric Titration.
From hyprowira.com
Understanding Permanganometric Titration and How It Works Indicator Used In Permanganometric Titration Permanganate requires low ph, or presence of strong acid. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Oxidation of fe 2+ by permanganate is one of the most popular. This is a procedure which depends upon measurement of the e.m.f. Between a reference electrode and an. Indicator Used In Permanganometric Titration.
From theedge.com.hk
Chemistry How To Titration The Edge Indicator Used In Permanganometric Titration This is a procedure which depends upon measurement of the e.m.f. In an indicator based titration you add another. Permanganate requires low ph, or presence of strong acid. Between a reference electrode and an indicator (redox) electrode at. There are two basic types of acid base titrations, indicator and potentiometric. Generally for the titration process, external indicators are used to. Indicator Used In Permanganometric Titration.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Indicator Used In Permanganometric Titration The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. In an indicator based titration you add another. In close proximity to the endpoint, the action of the indicator is. Best results can be obtained when permanganate is used for. Generally for the titration process, external indicators are. Indicator Used In Permanganometric Titration.
From pubs.acs.org
Permanganometric Titration for the Quantification of Purified Bis(2,4,4 Indicator Used In Permanganometric Titration Oxidation of fe 2+ by permanganate is one of the most popular. In an indicator based titration you add another. In close proximity to the endpoint, the action of the indicator is. There are two basic types of acid base titrations, indicator and potentiometric. This is a procedure which depends upon measurement of the e.m.f. Best results can be obtained. Indicator Used In Permanganometric Titration.
From www.researchgate.net
(PDF) Permanganometric Titration for the Quantification of Purified Bis Indicator Used In Permanganometric Titration This is a procedure which depends upon measurement of the e.m.f. Generally for the titration process, external indicators are used to predict the completion of a titration process, but in the case of potassium permanganate titration, kmno 4 acts as a self. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an. Indicator Used In Permanganometric Titration.
From mungfali.com
Acid Base Titration Indicator Indicator Used In Permanganometric Titration There are two basic types of acid base titrations, indicator and potentiometric. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Oxidation of fe 2+ by permanganate is one of the most popular. Best results can be obtained when permanganate is used for. Permanganometric titration of iron. Indicator Used In Permanganometric Titration.
From slideplayer.com
Acid Base Titration Curves & Indicators ppt download Indicator Used In Permanganometric Titration Generally for the titration process, external indicators are used to predict the completion of a titration process, but in the case of potassium permanganate titration, kmno 4 acts as a self. Oxidation of fe 2+ by permanganate is one of the most popular. Permanganometric titration of iron (ii) general remarks. Permanganate requires low ph, or presence of strong acid. The. Indicator Used In Permanganometric Titration.
From www.vrogue.co
Titration Indicator Types Procedure Indicators vrogue.co Indicator Used In Permanganometric Titration Permanganate requires low ph, or presence of strong acid. This is a procedure which depends upon measurement of the e.m.f. Between a reference electrode and an indicator (redox) electrode at. In an indicator based titration you add another. There are two basic types of acid base titrations, indicator and potentiometric. Best results can be obtained when permanganate is used for.. Indicator Used In Permanganometric Titration.
From psu.pb.unizin.org
AcidBase Titrations (14.7) Chemistry 110 Indicator Used In Permanganometric Titration There are two basic types of acid base titrations, indicator and potentiometric. Permanganate requires low ph, or presence of strong acid. This is a procedure which depends upon measurement of the e.m.f. Between a reference electrode and an indicator (redox) electrode at. In close proximity to the endpoint, the action of the indicator is. Oxidation of fe 2+ by permanganate. Indicator Used In Permanganometric Titration.
From pubs.acs.org
Permanganometric Titration for the Quantification of Purified Bis(2,4,4 Indicator Used In Permanganometric Titration Permanganometric titration of iron (ii) general remarks. Best results can be obtained when permanganate is used for. Oxidation of fe 2+ by permanganate is one of the most popular. Permanganate requires low ph, or presence of strong acid. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration.. Indicator Used In Permanganometric Titration.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Indicator Used In Permanganometric Titration In close proximity to the endpoint, the action of the indicator is. There are two basic types of acid base titrations, indicator and potentiometric. Oxidation of fe 2+ by permanganate is one of the most popular. Generally for the titration process, external indicators are used to predict the completion of a titration process, but in the case of potassium permanganate. Indicator Used In Permanganometric Titration.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Indicator Used In Permanganometric Titration Best results can be obtained when permanganate is used for. Permanganometric titration of iron (ii) general remarks. Oxidation of fe 2+ by permanganate is one of the most popular. Permanganate requires low ph, or presence of strong acid. There are two basic types of acid base titrations, indicator and potentiometric. Between a reference electrode and an indicator (redox) electrode at.. Indicator Used In Permanganometric Titration.
From www.slideserve.com
PPT Indicators for AcidBase Titrations (Sec. 96) PowerPoint Indicator Used In Permanganometric Titration In close proximity to the endpoint, the action of the indicator is. Permanganate requires low ph, or presence of strong acid. In an indicator based titration you add another. This is a procedure which depends upon measurement of the e.m.f. There are two basic types of acid base titrations, indicator and potentiometric. Permanganometric titration of iron (ii) general remarks. Oxidation. Indicator Used In Permanganometric Titration.
From www.slideserve.com
PPT Acid Base Review PowerPoint Presentation, free download ID2814652 Indicator Used In Permanganometric Titration Generally for the titration process, external indicators are used to predict the completion of a titration process, but in the case of potassium permanganate titration, kmno 4 acts as a self. Between a reference electrode and an indicator (redox) electrode at. In close proximity to the endpoint, the action of the indicator is. Oxidation of fe 2+ by permanganate is. Indicator Used In Permanganometric Titration.
From gioteyspe.blob.core.windows.net
Indicator Used For Complexometric Titrations at Jerlene Powell blog Indicator Used In Permanganometric Titration Generally for the titration process, external indicators are used to predict the completion of a titration process, but in the case of potassium permanganate titration, kmno 4 acts as a self. Between a reference electrode and an indicator (redox) electrode at. Permanganometric titration of iron (ii) general remarks. Best results can be obtained when permanganate is used for. Oxidation of. Indicator Used In Permanganometric Titration.
From www.slideshare.net
Acid base titration Indicator Used In Permanganometric Titration In close proximity to the endpoint, the action of the indicator is. In an indicator based titration you add another. Generally for the titration process, external indicators are used to predict the completion of a titration process, but in the case of potassium permanganate titration, kmno 4 acts as a self. This is a procedure which depends upon measurement of. Indicator Used In Permanganometric Titration.
From www.slideshare.net
1 Titrations Indicator Used In Permanganometric Titration The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Permanganometric titration of iron (ii) general remarks. In an indicator based titration you add another. Permanganate requires low ph, or presence of strong acid. Generally for the titration process, external indicators are used to predict the completion of. Indicator Used In Permanganometric Titration.
From www.studypool.com
SOLUTION Indicators used in titration Studypool Indicator Used In Permanganometric Titration Between a reference electrode and an indicator (redox) electrode at. There are two basic types of acid base titrations, indicator and potentiometric. This is a procedure which depends upon measurement of the e.m.f. Permanganate requires low ph, or presence of strong acid. Oxidation of fe 2+ by permanganate is one of the most popular. In close proximity to the endpoint,. Indicator Used In Permanganometric Titration.
From www.studypool.com
SOLUTION Indicators used in titration Studypool Indicator Used In Permanganometric Titration In close proximity to the endpoint, the action of the indicator is. There are two basic types of acid base titrations, indicator and potentiometric. Between a reference electrode and an indicator (redox) electrode at. Generally for the titration process, external indicators are used to predict the completion of a titration process, but in the case of potassium permanganate titration, kmno. Indicator Used In Permanganometric Titration.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Indicator Used In Permanganometric Titration This is a procedure which depends upon measurement of the e.m.f. There are two basic types of acid base titrations, indicator and potentiometric. Permanganometric titration of iron (ii) general remarks. Between a reference electrode and an indicator (redox) electrode at. In close proximity to the endpoint, the action of the indicator is. Oxidation of fe 2+ by permanganate is one. Indicator Used In Permanganometric Titration.
From www.science-revision.co.uk
Titrations Indicator Used In Permanganometric Titration The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Best results can be obtained when permanganate is used for. Permanganometric titration of iron (ii) general remarks. This is a procedure which depends upon measurement of the e.m.f. There are two basic types of acid base titrations, indicator. Indicator Used In Permanganometric Titration.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Indicator Used In Permanganometric Titration In an indicator based titration you add another. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Between a reference electrode and an indicator (redox) electrode at. This is a procedure which depends upon measurement of the e.m.f. There are two basic types of acid base titrations,. Indicator Used In Permanganometric Titration.
From www.studypool.com
SOLUTION Indicators used in titration Studypool Indicator Used In Permanganometric Titration There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another. Permanganometric titration of iron (ii) general remarks. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. This is a procedure which depends upon measurement of the e.m.f.. Indicator Used In Permanganometric Titration.
From hxevhdmch.blob.core.windows.net
What Is A Titration Indicator at Kelly Ayala blog Indicator Used In Permanganometric Titration In an indicator based titration you add another. In close proximity to the endpoint, the action of the indicator is. Permanganometric titration of iron (ii) general remarks. This is a procedure which depends upon measurement of the e.m.f. Best results can be obtained when permanganate is used for. There are two basic types of acid base titrations, indicator and potentiometric.. Indicator Used In Permanganometric Titration.