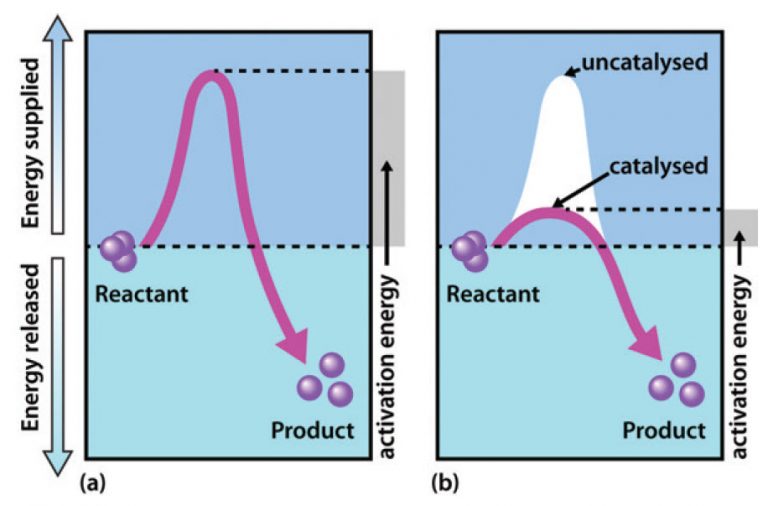
Catalysts Change The Energy Of . Catalysts (including enzymes) speed up chemical reactions. energy changes can be represented using energy profiles. catalysts and activation energy to increase the rate of a reaction you need to increase the number of successful collisions. catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. revision notes on 7.1.4 catalysts for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. temperature, concentration, pressure and the use of catalysts affect reaction rate. Part of combined science rates of reaction and. In less energetic collisions, the particles just bounce off. the effect of a catalyst on the activation energy is shown on a chart called a. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. One possible way of doing this. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. reactions happen when molecules collide with at least enough energy to equal activation energy. Chart showing how the energy of.
from wou.edu
catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. catalysts and activation energy to increase the rate of a reaction you need to increase the number of successful collisions. revision notes on 7.1.4 catalysts for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. Part of combined science rates of reaction and. In less energetic collisions, the particles just bounce off. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. temperature, concentration, pressure and the use of catalysts affect reaction rate. the effect of a catalyst on the activation energy is shown on a chart called a. Catalysts (including enzymes) speed up chemical reactions.
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry
Catalysts Change The Energy Of One possible way of doing this. revision notes on 7.1.4 catalysts for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. catalysts and activation energy to increase the rate of a reaction you need to increase the number of successful collisions. Chart showing how the energy of. reactions happen when molecules collide with at least enough energy to equal activation energy. Catalysts (including enzymes) speed up chemical reactions. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. the effect of a catalyst on the activation energy is shown on a chart called a. One possible way of doing this. In less energetic collisions, the particles just bounce off. temperature, concentration, pressure and the use of catalysts affect reaction rate. Part of combined science rates of reaction and. energy changes can be represented using energy profiles.
From www.thoughtco.com
Catalysis Definition in Chemistry Catalysts Change The Energy Of Catalysts (including enzymes) speed up chemical reactions. One possible way of doing this. Part of combined science rates of reaction and. the effect of a catalyst on the activation energy is shown on a chart called a. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. energy. Catalysts Change The Energy Of.
From chem.libretexts.org
6.10 Energies, and Catalysts Chemistry LibreTexts Catalysts Change The Energy Of catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. energy changes can be represented using energy profiles. temperature, concentration, pressure and the use of catalysts affect reaction rate. revision notes on 7.1.4 catalysts for the edexcel gcse chemistry syllabus, written by the chemistry experts at save. Catalysts Change The Energy Of.
From www.chim.lu
Activation energy and catalysis Catalysts Change The Energy Of Part of combined science rates of reaction and. One possible way of doing this. the effect of a catalyst on the activation energy is shown on a chart called a. Chart showing how the energy of. energy changes can be represented using energy profiles. catalysts and activation energy to increase the rate of a reaction you need. Catalysts Change The Energy Of.
From www.waca.msf.org
Lesson Explainer Catalysts, Catalyst Catalysts Change The Energy Of One possible way of doing this. reactions happen when molecules collide with at least enough energy to equal activation energy. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts (including enzymes) speed up chemical reactions. revision notes on 7.1.4 catalysts for the edexcel gcse chemistry syllabus,. Catalysts Change The Energy Of.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalysts Change The Energy Of In less energetic collisions, the particles just bounce off. Catalysts (including enzymes) speed up chemical reactions. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. the effect of a catalyst on the. Catalysts Change The Energy Of.
From courses.lumenlearning.com
Catalysis Chemistry Catalysts Change The Energy Of catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Chart showing how the energy of. catalysts and activation energy to increase the rate of a reaction you need to increase the number of successful collisions. catalysts function by allowing the reaction to take place through an alternative. Catalysts Change The Energy Of.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Catalysts Change The Energy Of Chart showing how the energy of. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. reactions happen when molecules collide with at least enough energy to equal activation energy. energy changes can be represented using energy profiles. catalysts and activation energy to increase the rate of. Catalysts Change The Energy Of.
From www.slideserve.com
PPT Outline of Enzymes PowerPoint Presentation, free download ID364722 Catalysts Change The Energy Of Catalysts (including enzymes) speed up chemical reactions. temperature, concentration, pressure and the use of catalysts affect reaction rate. In less energetic collisions, the particles just bounce off. Part of combined science rates of reaction and. Chart showing how the energy of. catalysts and activation energy to increase the rate of a reaction you need to increase the number. Catalysts Change The Energy Of.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalysts Change The Energy Of Part of combined science rates of reaction and. In less energetic collisions, the particles just bounce off. One possible way of doing this. reactions happen when molecules collide with at least enough energy to equal activation energy. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In heterogeneous. Catalysts Change The Energy Of.
From www.nagwa.com
Question Video Determining How Catalysts Affect the Energy Changes Catalysts Change The Energy Of One possible way of doing this. catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. Chart showing how the energy of. reactions happen when molecules collide with at least enough energy to equal activation energy. energy changes can be represented using energy profiles. In heterogeneous catalysis, catalysts. Catalysts Change The Energy Of.
From www.slideserve.com
PPT Reaction PowerPoint Presentation, free download ID1835084 Catalysts Change The Energy Of In less energetic collisions, the particles just bounce off. Part of combined science rates of reaction and. Chart showing how the energy of. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. One. Catalysts Change The Energy Of.
From dxooagcgl.blob.core.windows.net
How Does The Presence Of A Catalyst Affect The Activation Energy Of A Catalysts Change The Energy Of the effect of a catalyst on the activation energy is shown on a chart called a. One possible way of doing this. catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. catalysts and activation energy to increase the rate of a reaction you need to increase the. Catalysts Change The Energy Of.
From schoolbag.info
A catalyst speeds up a reaction by providing the reactants with an Catalysts Change The Energy Of Chart showing how the energy of. catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. Part of combined science rates of reaction and. energy changes can be represented using energy profiles. catalysts and activation energy to increase the rate of a reaction you need to increase the. Catalysts Change The Energy Of.
From 2012books.lardbucket.org
Catalysis Catalysts Change The Energy Of catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. Chart showing how the energy of. reactions happen when molecules collide with at least enough energy to equal activation. Catalysts Change The Energy Of.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram Catalysts Change The Energy Of energy changes can be represented using energy profiles. temperature, concentration, pressure and the use of catalysts affect reaction rate. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. In less energetic collisions, the particles just bounce off. Catalysts (including enzymes) speed up chemical reactions. One possible way of doing this. Chart. Catalysts Change The Energy Of.
From dxooagcgl.blob.core.windows.net
How Does The Presence Of A Catalyst Affect The Activation Energy Of A Catalysts Change The Energy Of catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. catalysts and activation energy to increase the rate of a reaction you need to increase the number of successful collisions. catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation. Catalysts Change The Energy Of.
From www.pnnl.gov
Catalysis PNNL Catalysts Change The Energy Of In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. Part of combined science rates of reaction and. Chart showing how the energy of. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. reactions happen when molecules collide with at least enough. Catalysts Change The Energy Of.
From www.youtube.com
Catalyst Affects Reaction Rate Energy Diagram with a Catalyst YouTube Catalysts Change The Energy Of energy changes can be represented using energy profiles. In less energetic collisions, the particles just bounce off. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. Part of combined science rates of reaction and. revision notes on 7.1.4 catalysts for the edexcel gcse chemistry syllabus, written by the chemistry experts at. Catalysts Change The Energy Of.
From chemwiki.ucdavis.edu
Catalytic Hydrogenation of Alkenes Chemwiki Catalysts Change The Energy Of In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. energy changes can be represented using energy profiles. One possible way of doing this. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. temperature, concentration, pressure and the use of catalysts. Catalysts Change The Energy Of.
From www.cheric.org
Chemical Reaction (Reaction rate) Catalysts Change The Energy Of reactions happen when molecules collide with at least enough energy to equal activation energy. One possible way of doing this. In less energetic collisions, the particles just bounce off. Part of combined science rates of reaction and. Chart showing how the energy of. catalysts function by allowing the reaction to take place through an alternative mechanism that requires. Catalysts Change The Energy Of.
From www.chemistrylearner.com
Activation Energy Definition, Formula, and Graph Catalysts Change The Energy Of energy changes can be represented using energy profiles. One possible way of doing this. temperature, concentration, pressure and the use of catalysts affect reaction rate. revision notes on 7.1.4 catalysts for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. catalysts and activation energy to increase the rate of a reaction. Catalysts Change The Energy Of.
From www.researchgate.net
Free energy of activation of uncatalyzed and catalyzed reactions Catalysts Change The Energy Of One possible way of doing this. revision notes on 7.1.4 catalysts for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. Catalysts (including enzymes) speed up chemical reactions. catalysts and activation energy to increase the rate of a reaction you need to increase the number of successful collisions. catalysts allow a reaction. Catalysts Change The Energy Of.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalysts Change The Energy Of revision notes on 7.1.4 catalysts for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. the effect of a catalyst on the activation energy is shown on a chart called a. catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. In less. Catalysts Change The Energy Of.
From circuitdbplastered.z13.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalysts Change The Energy Of Chart showing how the energy of. the effect of a catalyst on the activation energy is shown on a chart called a. Part of combined science rates of reaction and. revision notes on 7.1.4 catalysts for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. reactions happen when molecules collide with at. Catalysts Change The Energy Of.
From www.chemistrystudent.com
Boltzmann Distribution Curves (ALevel) ChemistryStudent Catalysts Change The Energy Of revision notes on 7.1.4 catalysts for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. Catalysts (including enzymes) speed up chemical reactions. Part of combined science rates of reaction and. catalysts and activation energy to increase the rate of a reaction you need to increase the number of successful collisions. reactions happen. Catalysts Change The Energy Of.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2568751 Catalysts Change The Energy Of revision notes on 7.1.4 catalysts for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. energy changes can be represented using energy profiles. Catalysts (including enzymes) speed up chemical reactions. temperature, concentration, pressure and the use of catalysts affect reaction rate. the effect of a catalyst on the activation energy is. Catalysts Change The Energy Of.
From dxooagcgl.blob.core.windows.net
How Does The Presence Of A Catalyst Affect The Activation Energy Of A Catalysts Change The Energy Of energy changes can be represented using energy profiles. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. Chart showing how the energy of. the effect of a catalyst on the activation energy is shown on a chart called a. catalysts and activation energy to increase the rate of a reaction. Catalysts Change The Energy Of.
From blog.syrris.com
Solid phase catalysis in continuous flow Syrris chemistry blog Catalysts Change The Energy Of catalysts and activation energy to increase the rate of a reaction you need to increase the number of successful collisions. energy changes can be represented using energy profiles. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. revision notes on 7.1.4 catalysts for the edexcel gcse chemistry syllabus, written by. Catalysts Change The Energy Of.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID3558810 Catalysts Change The Energy Of energy changes can be represented using energy profiles. catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. One possible way of doing this. Part of combined science rates of reaction and. In less energetic collisions, the particles just bounce off. revision notes on 7.1.4 catalysts for the. Catalysts Change The Energy Of.
From scitechdaily.com
Science Made Simple What Are Catalysts? Catalysts Change The Energy Of reactions happen when molecules collide with at least enough energy to equal activation energy. Chart showing how the energy of. revision notes on 7.1.4 catalysts for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. In less energetic collisions, the particles just bounce off. catalysts allow a reaction to proceed via a. Catalysts Change The Energy Of.
From www.catalystseurope.org
What are catalysts? Catalysts Change The Energy Of Chart showing how the energy of. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. revision notes on 7.1.4 catalysts for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. reactions happen when molecules collide with at least enough energy to equal. Catalysts Change The Energy Of.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalysts Change The Energy Of reactions happen when molecules collide with at least enough energy to equal activation energy. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. energy changes can be represented using energy profiles. catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy.. Catalysts Change The Energy Of.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Catalysts Change The Energy Of Part of combined science rates of reaction and. reactions happen when molecules collide with at least enough energy to equal activation energy. energy changes can be represented using energy profiles. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Chart showing how the energy of. catalysts. Catalysts Change The Energy Of.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Catalysts Change The Energy Of catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. the effect of. Catalysts Change The Energy Of.
From guidefixcasiranilv.z4.web.core.windows.net
How To Read Energy Diagrams Chemistry Catalysts Change The Energy Of Part of combined science rates of reaction and. temperature, concentration, pressure and the use of catalysts affect reaction rate. One possible way of doing this. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. energy changes can be represented using energy profiles. catalysts allow a reaction to proceed via a. Catalysts Change The Energy Of.