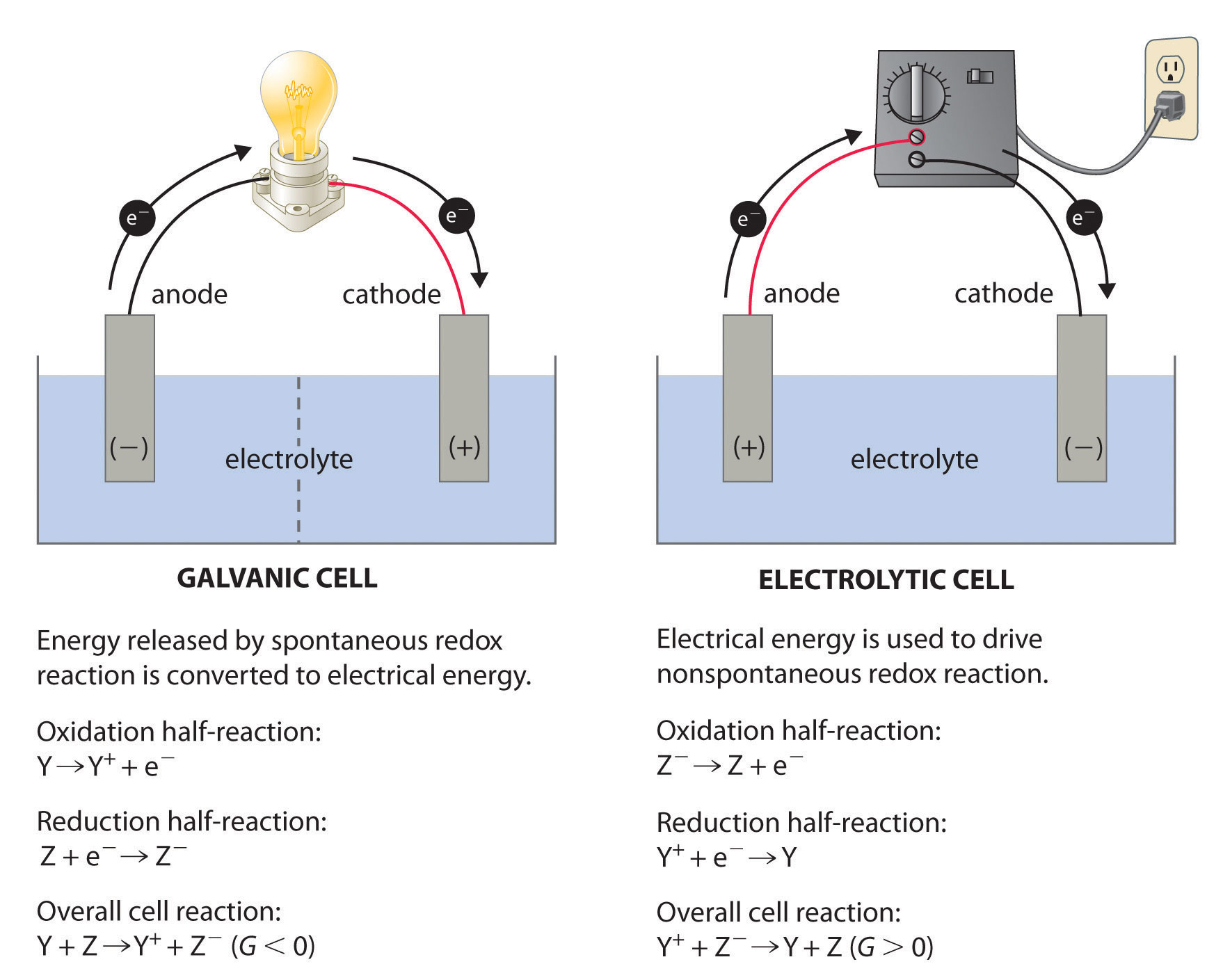
Electrochemistry Concentration Cell . applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and the concentrations of the dissolved ions change. concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. using the nernst equation to calculate cell potential for a concentration. this chemistry video tutorial provides a basic introduction into.
from saylordotorg.github.io
concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. using the nernst equation to calculate cell potential for a concentration. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and the concentrations of the dissolved ions change. this chemistry video tutorial provides a basic introduction into.
Electrochemistry
Electrochemistry Concentration Cell using the nernst equation to calculate cell potential for a concentration. concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and the concentrations of the dissolved ions change. using the nernst equation to calculate cell potential for a concentration. this chemistry video tutorial provides a basic introduction into.
From www.youtube.com
KAC32.7 Electrochemistry Effect of Concentration on Cell Potentials YouTube Electrochemistry Concentration Cell applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and the concentrations of the dissolved ions change. using the nernst equation to calculate cell potential for a concentration. concentration cells are composed of the same electrode and electrolyte solution. Electrochemistry Concentration Cell.
From www.youtube.com
DAT Electrochemistry Concentration Cell, Nernst Equation YouTube Electrochemistry Concentration Cell this chemistry video tutorial provides a basic introduction into. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and the concentrations of the dissolved ions change. concentration cells are composed of the same electrode and electrolyte solution with different. Electrochemistry Concentration Cell.
From saylordotorg.github.io
Electrochemistry Electrochemistry Concentration Cell applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and the concentrations of the dissolved ions change. this chemistry video tutorial provides a basic introduction into. using the nernst equation to calculate cell potential for a concentration. concentration. Electrochemistry Concentration Cell.
From slidetodoc.com
Electrochemistry Electrochemistry the study of the interchange of Electrochemistry Concentration Cell concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and the concentrations of the dissolved ions change. using the nernst. Electrochemistry Concentration Cell.
From cider.uoregon.edu
Electrochemical Cells Concentration Cells Demonstration and Simulation CIDER Electrochemistry Concentration Cell using the nernst equation to calculate cell potential for a concentration. concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. this chemistry video tutorial provides a basic introduction into. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to. Electrochemistry Concentration Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2793783 Electrochemistry Concentration Cell concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. this chemistry video tutorial provides a basic introduction into. using the nernst equation to calculate cell potential for a concentration. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to. Electrochemistry Concentration Cell.
From www.differencebetween.com
Difference Between Concentration Cell and Chemical Cell Compare the Difference Between Similar Electrochemistry Concentration Cell applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and the concentrations of the dissolved ions change. this chemistry video tutorial provides a basic introduction into. using the nernst equation to calculate cell potential for a concentration. concentration. Electrochemistry Concentration Cell.
From cider.uoregon.edu
Electrochemical Cells Concentration Cells Demonstration and Simulation CIDER Electrochemistry Concentration Cell applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and the concentrations of the dissolved ions change. concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. this chemistry video. Electrochemistry Concentration Cell.
From www.youtube.com
Lec31 Amalgam Electrode concentration cell• EMF derivation Electrochemistry BSc, MSc, IIT Electrochemistry Concentration Cell using the nernst equation to calculate cell potential for a concentration. concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses. Electrochemistry Concentration Cell.
From www.youtube.com
Electrochemistry Lecture 5 (Electrode & Electrolyte Concentration Cells) YouTube Electrochemistry Concentration Cell using the nernst equation to calculate cell potential for a concentration. concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses. Electrochemistry Concentration Cell.
From www.youtube.com
Electrochemistry The Concentration Cell. YouTube Electrochemistry Concentration Cell applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and the concentrations of the dissolved ions change. concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. this chemistry video. Electrochemistry Concentration Cell.
From www.youtube.com
Lec29 Concentration Cells Types of Concentration Cells Electrochemistry BSc, MSc, IIT JAM Electrochemistry Concentration Cell using the nernst equation to calculate cell potential for a concentration. concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses. Electrochemistry Concentration Cell.
From www.youtube.com
Electrochemistry Lecture 6 (Electrolyte Concentration Cells Without Transference) YouTube Electrochemistry Concentration Cell applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and the concentrations of the dissolved ions change. using the nernst equation to calculate cell potential for a concentration. this chemistry video tutorial provides a basic introduction into. concentration. Electrochemistry Concentration Cell.
From www.scribd.com
Electrochemistry Concentration Cell PDF Ion Redox Electrochemistry Concentration Cell concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. using the nernst equation to calculate cell potential for a concentration. this chemistry video tutorial provides a basic introduction into. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to. Electrochemistry Concentration Cell.
From chemistry.stackexchange.com
electrochemistry What is the physical explanation driving current flow in a concentration cell Electrochemistry Concentration Cell this chemistry video tutorial provides a basic introduction into. concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. using the nernst equation to calculate cell potential for a concentration. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to. Electrochemistry Concentration Cell.
From saylordotorg.github.io
Electrochemistry Electrochemistry Concentration Cell applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and the concentrations of the dissolved ions change. concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. using the nernst. Electrochemistry Concentration Cell.
From cider.uoregon.edu
Electrochemical Cells Concentration Cells Demonstration and Simulation CIDER Electrochemistry Concentration Cell using the nernst equation to calculate cell potential for a concentration. this chemistry video tutorial provides a basic introduction into. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and the concentrations of the dissolved ions change. concentration. Electrochemistry Concentration Cell.
From www.youtube.com
Concentration Cell in Electrochemistry Electrochemistry 2 YouTube Electrochemistry Concentration Cell applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and the concentrations of the dissolved ions change. using the nernst equation to calculate cell potential for a concentration. concentration cells are composed of the same electrode and electrolyte solution. Electrochemistry Concentration Cell.
From www.youtube.com
Concentration Cell Electrochemistry Chemistry Allen Digital YouTube Electrochemistry Concentration Cell this chemistry video tutorial provides a basic introduction into. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and the concentrations of the dissolved ions change. concentration cells are composed of the same electrode and electrolyte solution with different. Electrochemistry Concentration Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2281241 Electrochemistry Concentration Cell using the nernst equation to calculate cell potential for a concentration. concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. this chemistry video tutorial provides a basic introduction into. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to. Electrochemistry Concentration Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2851831 Electrochemistry Concentration Cell concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. using the nernst equation to calculate cell potential for a concentration. this chemistry video tutorial provides a basic introduction into. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to. Electrochemistry Concentration Cell.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free download ID889288 Electrochemistry Concentration Cell applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and the concentrations of the dissolved ions change. this chemistry video tutorial provides a basic introduction into. concentration cells are composed of the same electrode and electrolyte solution with different. Electrochemistry Concentration Cell.
From saylordotorg.github.io
Electrochemistry Electrochemistry Concentration Cell this chemistry video tutorial provides a basic introduction into. concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and the. Electrochemistry Concentration Cell.
From schoolbag.info
Figure 12.3. The Cell Membrane as an Example of a Concentration Cell The electrochemical Electrochemistry Concentration Cell using the nernst equation to calculate cell potential for a concentration. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and the concentrations of the dissolved ions change. this chemistry video tutorial provides a basic introduction into. concentration. Electrochemistry Concentration Cell.
From circuittawnilynne2461.z14.web.core.windows.net
Electrochemistry Cell Diagram Electrochemistry Concentration Cell applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and the concentrations of the dissolved ions change. this chemistry video tutorial provides a basic introduction into. concentration cells are composed of the same electrode and electrolyte solution with different. Electrochemistry Concentration Cell.
From www.slideserve.com
PPT Electrochemical methods of analysis PowerPoint Presentation, free download ID9238757 Electrochemistry Concentration Cell applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and the concentrations of the dissolved ions change. using the nernst equation to calculate cell potential for a concentration. concentration cells are composed of the same electrode and electrolyte solution. Electrochemistry Concentration Cell.
From cafecentralmugron.fr
Lab 13 Electrochemistry And The Nernst Equation Electrochemistry Concentration Cell applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and the concentrations of the dissolved ions change. using the nernst equation to calculate cell potential for a concentration. concentration cells are composed of the same electrode and electrolyte solution. Electrochemistry Concentration Cell.
From www.scribd.com
Electrochemistry Concentration Cells PDF Ph Corrosion Electrochemistry Concentration Cell concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. using the nernst equation to calculate cell potential for a concentration. this chemistry video tutorial provides a basic introduction into. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to. Electrochemistry Concentration Cell.
From www.careerpower.in
Class 12 Electrochemistry Electrochemical Series, Concentration Cell, Battery Electrochemistry Concentration Cell this chemistry video tutorial provides a basic introduction into. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and the concentrations of the dissolved ions change. using the nernst equation to calculate cell potential for a concentration. concentration. Electrochemistry Concentration Cell.
From general.chemistrysteps.com
Concentration Cells Chemistry Steps Electrochemistry Concentration Cell using the nernst equation to calculate cell potential for a concentration. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and the concentrations of the dissolved ions change. concentration cells are composed of the same electrode and electrolyte solution. Electrochemistry Concentration Cell.
From www.slideserve.com
PPT Chemistry 142 Chapter 18 Electrochemistry PowerPoint Presentation ID1725688 Electrochemistry Concentration Cell applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and the concentrations of the dissolved ions change. concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. this chemistry video. Electrochemistry Concentration Cell.
From jackwestin.com
Concentration Cell Electrochemistry MCAT Content Electrochemistry Concentration Cell concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. this chemistry video tutorial provides a basic introduction into. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and the. Electrochemistry Concentration Cell.
From www.slideserve.com
PPT ELECTROCHEMISTRY PowerPoint Presentation, free download ID9339380 Electrochemistry Concentration Cell applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and the concentrations of the dissolved ions change. concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. this chemistry video. Electrochemistry Concentration Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID7041672 Electrochemistry Concentration Cell applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and the concentrations of the dissolved ions change. using the nernst equation to calculate cell potential for a concentration. this chemistry video tutorial provides a basic introduction into. concentration. Electrochemistry Concentration Cell.
From www.youtube.com
Electrochemistry II Nernst Equation, Concentration Cell YouTube Electrochemistry Concentration Cell concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. this chemistry video tutorial provides a basic introduction into. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and the. Electrochemistry Concentration Cell.