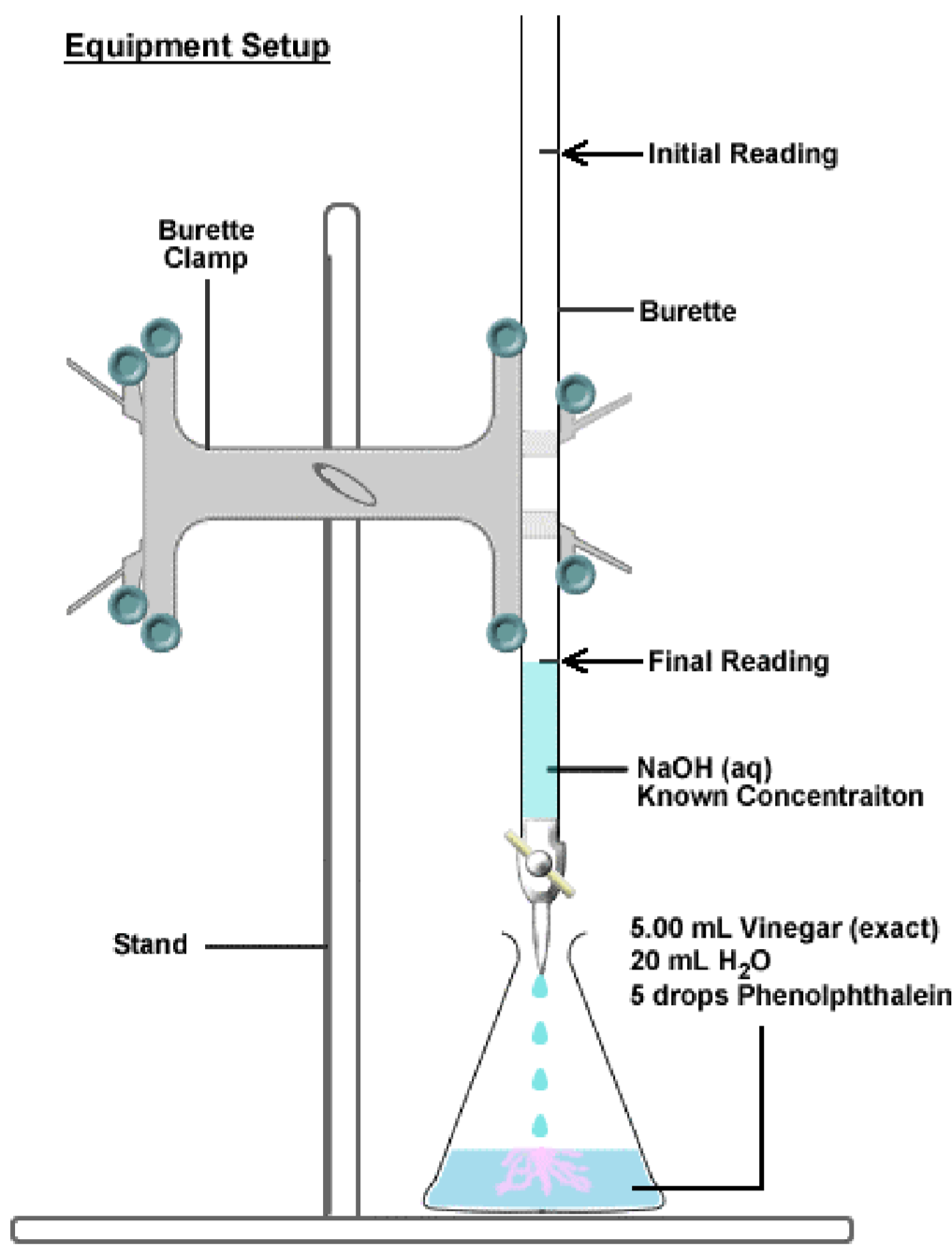
Titration Vinegar Lab . Microscale titration provides a fast and effective way to apply the principles of titration to the analysis of. you can visualize the process of an acid (vinegar) neutralization in a titration curve, which is a graph that shows the amount of added base (sodium hydroxide). click on the links below for lab 7, titration of vinegar: the incremental process of adding the ch3cooh solution is called titration. We will use titration to find. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. in this experiment we will perform a titration, a lab technique that is used to determine the concentration of a solute in a solution. It enables the concentration of the ch3cooh to be determined from the.
from chem.libretexts.org
in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. in this experiment we will perform a titration, a lab technique that is used to determine the concentration of a solute in a solution. We will use titration to find. the incremental process of adding the ch3cooh solution is called titration. It enables the concentration of the ch3cooh to be determined from the. click on the links below for lab 7, titration of vinegar: Microscale titration provides a fast and effective way to apply the principles of titration to the analysis of. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. you can visualize the process of an acid (vinegar) neutralization in a titration curve, which is a graph that shows the amount of added base (sodium hydroxide).
11 Titration of Vinegar (Experiment) Chemistry LibreTexts
Titration Vinegar Lab We will use titration to find. in this experiment we will perform a titration, a lab technique that is used to determine the concentration of a solute in a solution. It enables the concentration of the ch3cooh to be determined from the. the incremental process of adding the ch3cooh solution is called titration. Microscale titration provides a fast and effective way to apply the principles of titration to the analysis of. We will use titration to find. click on the links below for lab 7, titration of vinegar: in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. you can visualize the process of an acid (vinegar) neutralization in a titration curve, which is a graph that shows the amount of added base (sodium hydroxide). in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar.
From www.chegg.com
Solved TITRATION • CONCENTRATION OF VINEGAR INTRODUCTION Titration Vinegar Lab in this experiment we will perform a titration, a lab technique that is used to determine the concentration of a solute in a solution. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Microscale titration provides a fast and effective way to apply the principles of. Titration Vinegar Lab.
From www.chegg.com
Solved Prelaboratory Assignment Titration of Vinegar 1. In Titration Vinegar Lab in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. you can visualize the process of an acid (vinegar) neutralization in a titration curve, which is a graph that shows the amount of added base (sodium hydroxide). in this experiment, a technique known as a titration will be. Titration Vinegar Lab.
From www.chegg.com
Lab 8 Analysis of Vinegar by Titration This page Titration Vinegar Lab in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. the incremental process of adding the ch3cooh solution is called titration. you can visualize the process of an acid (vinegar) neutralization in a titration curve, which is a graph that shows the amount of added base. Titration Vinegar Lab.
From www.studypool.com
SOLUTION Titration analysis of vinegar lab Studypool Titration Vinegar Lab It enables the concentration of the ch3cooh to be determined from the. We will use titration to find. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. you can visualize the process of an acid (vinegar) neutralization in a titration curve, which is a graph that. Titration Vinegar Lab.
From www.youtube.com
Chemistry Lab Titration of Vinegar in Pickle Juice YouTube Titration Vinegar Lab in this experiment we will perform a titration, a lab technique that is used to determine the concentration of a solute in a solution. It enables the concentration of the ch3cooh to be determined from the. We will use titration to find. you can visualize the process of an acid (vinegar) neutralization in a titration curve, which is. Titration Vinegar Lab.
From www.studyxapp.com
titration concentration of vinegar introduction laboratory simulation lab data StudyX Titration Vinegar Lab We will use titration to find. Microscale titration provides a fast and effective way to apply the principles of titration to the analysis of. It enables the concentration of the ch3cooh to be determined from the. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. you. Titration Vinegar Lab.
From exokgqnrm.blob.core.windows.net
Titration Concentration Of Vinegar Lab at Kevin Dahlke blog Titration Vinegar Lab in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. We will use titration to find. you can visualize the process of an acid (vinegar) neutralization in a titration curve, which is a graph that shows the amount of added base (sodium hydroxide). in this experiment. Titration Vinegar Lab.
From www.chegg.com
Solved Titration for Acetic Acid in Vinegar Lab Report Titration Vinegar Lab We will use titration to find. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Microscale titration provides a fast and effective way to apply the principles of titration to the analysis of. click on the links below for lab 7, titration of vinegar: in. Titration Vinegar Lab.
From studylib.net
Titration of Vinegar Lab Titration Vinegar Lab It enables the concentration of the ch3cooh to be determined from the. the incremental process of adding the ch3cooh solution is called titration. in this experiment we will perform a titration, a lab technique that is used to determine the concentration of a solute in a solution. We will use titration to find. Microscale titration provides a fast. Titration Vinegar Lab.
From www.vrogue.co
11 Titration Of Vinegar Experiment Chemistry Libretex vrogue.co Titration Vinegar Lab Microscale titration provides a fast and effective way to apply the principles of titration to the analysis of. It enables the concentration of the ch3cooh to be determined from the. in this experiment we will perform a titration, a lab technique that is used to determine the concentration of a solute in a solution. in this experiment, a. Titration Vinegar Lab.
From www.chegg.com
Solved Week 6 Lab Titration Concentration of Vinegar Titration Vinegar Lab It enables the concentration of the ch3cooh to be determined from the. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. in this experiment we will perform a titration, a lab technique that is used to determine the concentration of a solute in a solution. . Titration Vinegar Lab.
From www.studypool.com
SOLUTION C Lab 6 Titration Of Acetic Acid In Vinegar Studypool Titration Vinegar Lab in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. you can visualize the process of an acid (vinegar) neutralization in a titration curve, which is a graph that shows the amount of added base (sodium hydroxide). click on the links below for lab 7, titration of vinegar:. Titration Vinegar Lab.
From studylib.net
Titration of Vinegar Titration Vinegar Lab We will use titration to find. click on the links below for lab 7, titration of vinegar: the incremental process of adding the ch3cooh solution is called titration. in this experiment we will perform a titration, a lab technique that is used to determine the concentration of a solute in a solution. you can visualize the. Titration Vinegar Lab.
From www.studypool.com
SOLUTION CHEM151 Phoenix Titration for Acetic Acid in Vinegar Assistant Lab Report Studypool Titration Vinegar Lab We will use titration to find. click on the links below for lab 7, titration of vinegar: in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. the incremental process of adding the ch3cooh solution is called titration. in this experiment we will perform a. Titration Vinegar Lab.
From www.chegg.com
Solved CHEMISTRY. TITRATION OF VINEGAR INTRODUCTION Titration Vinegar Lab in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. It enables the concentration of the ch3cooh to be determined from the. Microscale titration provides a fast and effective way to apply the principles of titration to the analysis of. in this experiment, a technique known as a titration. Titration Vinegar Lab.
From www.studypool.com
SOLUTION Titration of vinegar lab report Studypool Titration Vinegar Lab in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. in this experiment we will perform a titration, a lab technique that is used to determine the concentration of a solute in a solution. We will use titration to find. Microscale titration provides a fast and effective way to. Titration Vinegar Lab.
From www.scribd.com
Titration of Vinegar Experiment PDF Titration Chemistry Titration Vinegar Lab in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Microscale titration provides a fast and effective way to apply the principles of titration to the analysis of. in this experiment we will perform a titration, a lab technique that is used to determine the concentration of. Titration Vinegar Lab.
From www.chegg.com
Solved Titration Vinegar Lab It enables the concentration of the ch3cooh to be determined from the. the incremental process of adding the ch3cooh solution is called titration. click on the links below for lab 7, titration of vinegar: Microscale titration provides a fast and effective way to apply the principles of titration to the analysis of. in this experiment, a technique. Titration Vinegar Lab.
From chem4three.blogspot.com
CHEMISTRY 11 Titration of Vinegar LAB Titration Vinegar Lab It enables the concentration of the ch3cooh to be determined from the. click on the links below for lab 7, titration of vinegar: Microscale titration provides a fast and effective way to apply the principles of titration to the analysis of. you can visualize the process of an acid (vinegar) neutralization in a titration curve, which is a. Titration Vinegar Lab.
From www.studypool.com
SOLUTION Titration analysis of vinegar lab Studypool Titration Vinegar Lab We will use titration to find. Microscale titration provides a fast and effective way to apply the principles of titration to the analysis of. It enables the concentration of the ch3cooh to be determined from the. in this experiment we will perform a titration, a lab technique that is used to determine the concentration of a solute in a. Titration Vinegar Lab.
From www.chegg.com
Solved TITRATION. CONCENTRATION OF VINEGAR INTRODUCTION Titration Vinegar Lab in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. you can visualize the process of an acid (vinegar) neutralization in a titration curve, which is a graph that shows the amount of added base (sodium hydroxide). Microscale titration provides a fast and effective way to apply the principles. Titration Vinegar Lab.
From www.scribd.com
Lab Titration of Vinegar Acid Titration Titration Vinegar Lab you can visualize the process of an acid (vinegar) neutralization in a titration curve, which is a graph that shows the amount of added base (sodium hydroxide). in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. in this experiment, a technique known as a titration will be. Titration Vinegar Lab.
From exoyiwzey.blob.core.windows.net
Titration Lab With Vinegar And Naoh at Edward Gross blog Titration Vinegar Lab in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. click on the links below for lab 7, titration of vinegar: you can visualize the process of an acid (vinegar) neutralization in a titration curve, which is a graph that shows the amount of added base (sodium hydroxide).. Titration Vinegar Lab.
From www.coursehero.com
[Solved] Titration Vinegar Lab in this experiment we will perform a titration, a lab technique that is used to determine the concentration of a solute in a solution. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. We will use titration to find. the incremental process of adding the ch3cooh solution. Titration Vinegar Lab.
From www.chegg.com
Solved Lab Report Titration of Vinegar Experimental Data Titration Vinegar Lab We will use titration to find. click on the links below for lab 7, titration of vinegar: in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. the incremental process of adding the ch3cooh solution is called titration. It enables the concentration of the ch3cooh to. Titration Vinegar Lab.
From www.chegg.com
Solved TITRATION OF VINEGAR LAB REPORT SHOW ALL WORK (ATTACH Titration Vinegar Lab click on the links below for lab 7, titration of vinegar: We will use titration to find. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. It enables the concentration of the ch3cooh to be determined from the. in this experiment we will perform a titration, a. Titration Vinegar Lab.
From www.chegg.com
CHEMISTRY TITRATION OF VINEGAR INTRODUCTION Titration Vinegar Lab you can visualize the process of an acid (vinegar) neutralization in a titration curve, which is a graph that shows the amount of added base (sodium hydroxide). click on the links below for lab 7, titration of vinegar: It enables the concentration of the ch3cooh to be determined from the. We will use titration to find. in. Titration Vinegar Lab.
From www.scribd.com
Titration of Vinegar Lab Report PDF Titration Chemistry Titration Vinegar Lab It enables the concentration of the ch3cooh to be determined from the. We will use titration to find. click on the links below for lab 7, titration of vinegar: you can visualize the process of an acid (vinegar) neutralization in a titration curve, which is a graph that shows the amount of added base (sodium hydroxide). the. Titration Vinegar Lab.
From www.youtube.com
Vinegar Titration Part A & B General Chemistry Experiment YouTube Titration Vinegar Lab in this experiment we will perform a titration, a lab technique that is used to determine the concentration of a solute in a solution. Microscale titration provides a fast and effective way to apply the principles of titration to the analysis of. the incremental process of adding the ch3cooh solution is called titration. It enables the concentration of. Titration Vinegar Lab.
From www.chegg.com
Solved MISTRY. TITRATION OF VINEGAR RODUCTION LABORATORY Titration Vinegar Lab Microscale titration provides a fast and effective way to apply the principles of titration to the analysis of. you can visualize the process of an acid (vinegar) neutralization in a titration curve, which is a graph that shows the amount of added base (sodium hydroxide). We will use titration to find. It enables the concentration of the ch3cooh to. Titration Vinegar Lab.
From www.chegg.com
Solved REPORT.SHEETS Experiment 9 Titration of Vinegar Titration Vinegar Lab the incremental process of adding the ch3cooh solution is called titration. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. you can visualize the process of an acid (vinegar) neutralization in a titration curve, which is a graph that shows the amount of added base (sodium hydroxide).. Titration Vinegar Lab.
From www.youtube.com
CHM 132 / Lab 20 Titration of Vinegar YouTube Titration Vinegar Lab in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. We will use titration to find. It enables the concentration of the ch3cooh to be determined from the.. Titration Vinegar Lab.
From www.chegg.com
Solved CHEMISTRY. TITRATION OF VINEGAR NTRODUCTION Titration Vinegar Lab Microscale titration provides a fast and effective way to apply the principles of titration to the analysis of. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. in this experiment we will perform a titration, a lab technique that is used to determine the concentration of. Titration Vinegar Lab.
From studylib.net
The Titration of Acetic Acid in Vinegar Titration Vinegar Lab Microscale titration provides a fast and effective way to apply the principles of titration to the analysis of. in this experiment we will perform a titration, a lab technique that is used to determine the concentration of a solute in a solution. the incremental process of adding the ch3cooh solution is called titration. you can visualize the. Titration Vinegar Lab.
From chem.libretexts.org
11 Titration of Vinegar (Experiment) Chemistry LibreTexts Titration Vinegar Lab in this experiment we will perform a titration, a lab technique that is used to determine the concentration of a solute in a solution. you can visualize the process of an acid (vinegar) neutralization in a titration curve, which is a graph that shows the amount of added base (sodium hydroxide). the incremental process of adding the. Titration Vinegar Lab.