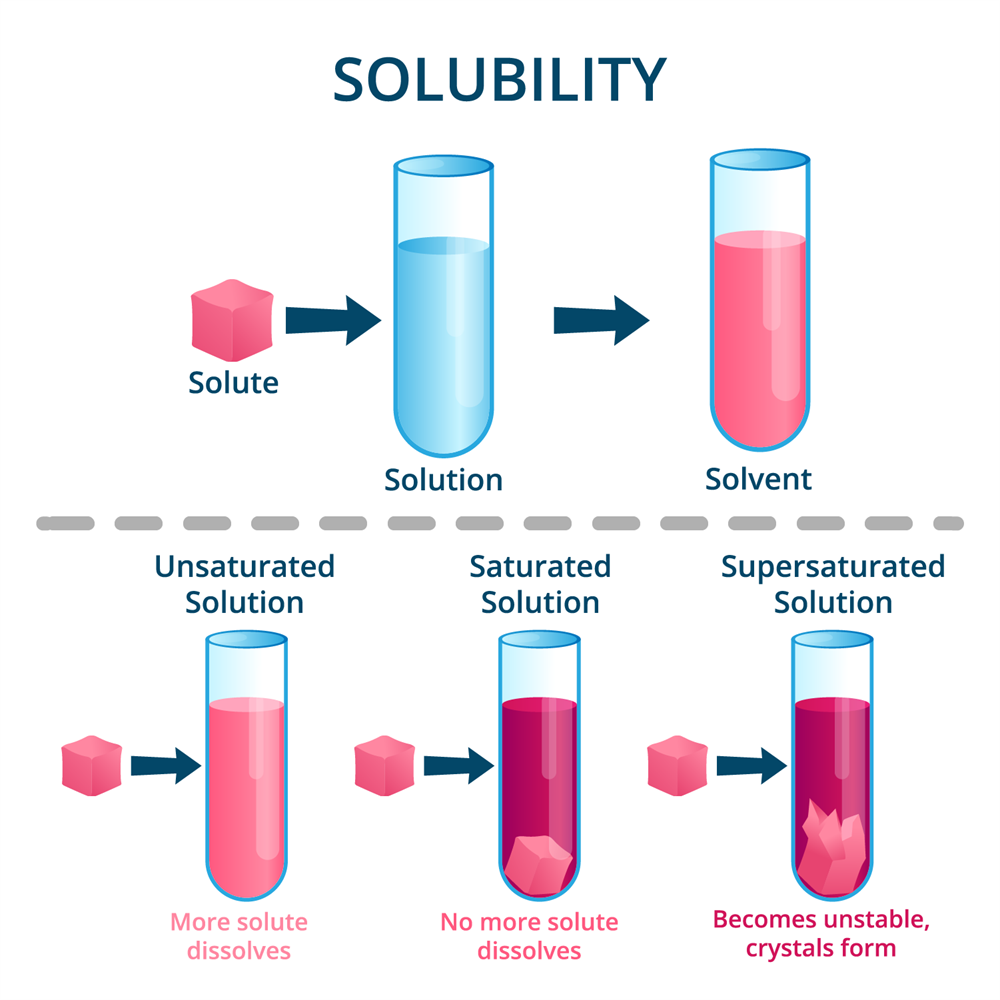
Dilute Solution Or Unsaturated . If more solute is added and it does not dissolve, then the original solution was saturated. A dilute solution is characterized by a low proportion of solute in the solvent, making it less concentrated. We will begin our discussion of solution concentration with two related and relative terms: Distinguish unsaturated, saturated, and supersaturated solutions. Dilute solutions have a lower density and lighter color compared to concentrated solutions, while unsaturated solutions have the ability to. The major component of the solution is called solvent, and the minor component (s) are called solute. How can you tell if a solution is saturated or unsaturated? Because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should be. A solution that contains a relatively low concentration of solute is called dilute, and one. A dilute solution is one. If both components in a solution are 50%, the term solute can be assigned to either component. If the solute’s concentration is less than its solubility, the solution is said to be unsaturated.
from www.yaclass.in
Dilute solutions have a lower density and lighter color compared to concentrated solutions, while unsaturated solutions have the ability to. If both components in a solution are 50%, the term solute can be assigned to either component. The major component of the solution is called solvent, and the minor component (s) are called solute. Distinguish unsaturated, saturated, and supersaturated solutions. If more solute is added and it does not dissolve, then the original solution was saturated. A dilute solution is one. We will begin our discussion of solution concentration with two related and relative terms: How can you tell if a solution is saturated or unsaturated? A solution that contains a relatively low concentration of solute is called dilute, and one. A dilute solution is characterized by a low proportion of solute in the solvent, making it less concentrated.
Types of solutions Based on the amount of the solute — lesson. Science
Dilute Solution Or Unsaturated The major component of the solution is called solvent, and the minor component (s) are called solute. The major component of the solution is called solvent, and the minor component (s) are called solute. We will begin our discussion of solution concentration with two related and relative terms: If the solute’s concentration is less than its solubility, the solution is said to be unsaturated. Because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should be. Distinguish unsaturated, saturated, and supersaturated solutions. If both components in a solution are 50%, the term solute can be assigned to either component. A solution that contains a relatively low concentration of solute is called dilute, and one. Dilute solutions have a lower density and lighter color compared to concentrated solutions, while unsaturated solutions have the ability to. A dilute solution is one. If more solute is added and it does not dissolve, then the original solution was saturated. A dilute solution is characterized by a low proportion of solute in the solvent, making it less concentrated. How can you tell if a solution is saturated or unsaturated?
From www.slideserve.com
PPT Unit 7 Solution Chemistry Chapter 13 PowerPoint Presentation Dilute Solution Or Unsaturated The major component of the solution is called solvent, and the minor component (s) are called solute. If the solute’s concentration is less than its solubility, the solution is said to be unsaturated. Because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should be. If more solute is added and. Dilute Solution Or Unsaturated.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Dilute Solution Or Unsaturated Dilute solutions have a lower density and lighter color compared to concentrated solutions, while unsaturated solutions have the ability to. Distinguish unsaturated, saturated, and supersaturated solutions. How can you tell if a solution is saturated or unsaturated? A dilute solution is characterized by a low proportion of solute in the solvent, making it less concentrated. If both components in a. Dilute Solution Or Unsaturated.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Dilute Solution Or Unsaturated The major component of the solution is called solvent, and the minor component (s) are called solute. Because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should be. A dilute solution is characterized by a low proportion of solute in the solvent, making it less concentrated. If the solute’s concentration. Dilute Solution Or Unsaturated.
From www.3ditalian.com
What is the Difference Between Dilute and Unsaturated Solution Dilute Solution Or Unsaturated If both components in a solution are 50%, the term solute can be assigned to either component. The major component of the solution is called solvent, and the minor component (s) are called solute. If more solute is added and it does not dissolve, then the original solution was saturated. We will begin our discussion of solution concentration with two. Dilute Solution Or Unsaturated.
From www.youtube.com
Dilute & Concentrated solution Saturated & Unsaturated solution Class Dilute Solution Or Unsaturated Distinguish unsaturated, saturated, and supersaturated solutions. If the solute’s concentration is less than its solubility, the solution is said to be unsaturated. A dilute solution is one. A dilute solution is characterized by a low proportion of solute in the solvent, making it less concentrated. A solution that contains a relatively low concentration of solute is called dilute, and one.. Dilute Solution Or Unsaturated.
From www.slideserve.com
PPT Unit 7 Solution Chemistry Chapter 13 PowerPoint Presentation Dilute Solution Or Unsaturated If the solute’s concentration is less than its solubility, the solution is said to be unsaturated. We will begin our discussion of solution concentration with two related and relative terms: A dilute solution is one. How can you tell if a solution is saturated or unsaturated? Distinguish unsaturated, saturated, and supersaturated solutions. The major component of the solution is called. Dilute Solution Or Unsaturated.
From www.youtube.com
Solutions Diluted, concentrated, Unsaturated, saturated solution Dilute Solution Or Unsaturated If both components in a solution are 50%, the term solute can be assigned to either component. The major component of the solution is called solvent, and the minor component (s) are called solute. A dilute solution is characterized by a low proportion of solute in the solvent, making it less concentrated. How can you tell if a solution is. Dilute Solution Or Unsaturated.
From www.youtube.com
Solutions Lesson 1 Solutions and Solubility YouTube Dilute Solution Or Unsaturated If more solute is added and it does not dissolve, then the original solution was saturated. The major component of the solution is called solvent, and the minor component (s) are called solute. We will begin our discussion of solution concentration with two related and relative terms: A dilute solution is characterized by a low proportion of solute in the. Dilute Solution Or Unsaturated.
From www.yaclass.in
Types of solutions Based on the amount of the solute — lesson. Science Dilute Solution Or Unsaturated If the solute’s concentration is less than its solubility, the solution is said to be unsaturated. If more solute is added and it does not dissolve, then the original solution was saturated. Distinguish unsaturated, saturated, and supersaturated solutions. We will begin our discussion of solution concentration with two related and relative terms: Dilute solutions have a lower density and lighter. Dilute Solution Or Unsaturated.
From www.youtube.com
CONCENTRATION OF SOLUTIONS SATURATED & UNSATURATED DILUTE Dilute Solution Or Unsaturated Because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should be. If both components in a solution are 50%, the term solute can be assigned to either component. If more solute is added and it does not dissolve, then the original solution was saturated. We will begin our discussion of. Dilute Solution Or Unsaturated.
From www.teachoo.com
Difference between Saturated and Unsaturated Solution Teachoo Dilute Solution Or Unsaturated Dilute solutions have a lower density and lighter color compared to concentrated solutions, while unsaturated solutions have the ability to. A dilute solution is characterized by a low proportion of solute in the solvent, making it less concentrated. Because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should be. If. Dilute Solution Or Unsaturated.
From sciencenotes.org
Supersaturated Solution Definition and Examples Dilute Solution Or Unsaturated If the solute’s concentration is less than its solubility, the solution is said to be unsaturated. A dilute solution is characterized by a low proportion of solute in the solvent, making it less concentrated. We will begin our discussion of solution concentration with two related and relative terms: Distinguish unsaturated, saturated, and supersaturated solutions. A dilute solution is one. Dilute. Dilute Solution Or Unsaturated.
From www.yourdictionary.com
Examples of Saturated Solution YourDictionary Dilute Solution Or Unsaturated The major component of the solution is called solvent, and the minor component (s) are called solute. If the solute’s concentration is less than its solubility, the solution is said to be unsaturated. If more solute is added and it does not dissolve, then the original solution was saturated. If both components in a solution are 50%, the term solute. Dilute Solution Or Unsaturated.
From slidetodoc.com
WATER AND SOLUTIONS A solution is a mixture Dilute Solution Or Unsaturated If both components in a solution are 50%, the term solute can be assigned to either component. If the solute’s concentration is less than its solubility, the solution is said to be unsaturated. Distinguish unsaturated, saturated, and supersaturated solutions. A dilute solution is one. A dilute solution is characterized by a low proportion of solute in the solvent, making it. Dilute Solution Or Unsaturated.
From www.youtube.com
Saturated Unsaturated and Supersaturated Solutions What is the Dilute Solution Or Unsaturated If the solute’s concentration is less than its solubility, the solution is said to be unsaturated. If both components in a solution are 50%, the term solute can be assigned to either component. Because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should be. If more solute is added and. Dilute Solution Or Unsaturated.
From solanolabs.com
Saturated solution definition chemistry Difference Between Saturated Dilute Solution Or Unsaturated A dilute solution is characterized by a low proportion of solute in the solvent, making it less concentrated. If the solute’s concentration is less than its solubility, the solution is said to be unsaturated. If more solute is added and it does not dissolve, then the original solution was saturated. Dilute solutions have a lower density and lighter color compared. Dilute Solution Or Unsaturated.
From www.slideserve.com
PPT Chapter 12 Solutions PowerPoint Presentation, free download ID Dilute Solution Or Unsaturated How can you tell if a solution is saturated or unsaturated? If both components in a solution are 50%, the term solute can be assigned to either component. Distinguish unsaturated, saturated, and supersaturated solutions. If more solute is added and it does not dissolve, then the original solution was saturated. We will begin our discussion of solution concentration with two. Dilute Solution Or Unsaturated.
From www.youtube.com
Dilute and Concentrated Solution YouTube Dilute Solution Or Unsaturated Because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should be. A dilute solution is characterized by a low proportion of solute in the solvent, making it less concentrated. If the solute’s concentration is less than its solubility, the solution is said to be unsaturated. Distinguish unsaturated, saturated, and supersaturated. Dilute Solution Or Unsaturated.
From www.numerade.com
SOLVED A solution can be both dilute and concentrated saturated und Dilute Solution Or Unsaturated Because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should be. We will begin our discussion of solution concentration with two related and relative terms: A solution that contains a relatively low concentration of solute is called dilute, and one. How can you tell if a solution is saturated or. Dilute Solution Or Unsaturated.
From www.slideserve.com
PPT Unit 6 Solutions PowerPoint Presentation, free download ID5505928 Dilute Solution Or Unsaturated Distinguish unsaturated, saturated, and supersaturated solutions. If the solute’s concentration is less than its solubility, the solution is said to be unsaturated. How can you tell if a solution is saturated or unsaturated? We will begin our discussion of solution concentration with two related and relative terms: A dilute solution is characterized by a low proportion of solute in the. Dilute Solution Or Unsaturated.
From www.slideserve.com
PPT Solutions and Solubility PowerPoint Presentation, free download Dilute Solution Or Unsaturated Distinguish unsaturated, saturated, and supersaturated solutions. We will begin our discussion of solution concentration with two related and relative terms: A solution that contains a relatively low concentration of solute is called dilute, and one. Dilute solutions have a lower density and lighter color compared to concentrated solutions, while unsaturated solutions have the ability to. If the solute’s concentration is. Dilute Solution Or Unsaturated.
From www.pinterest.com
saturated unsaturated and supersaturated solutions Google Search Dilute Solution Or Unsaturated A dilute solution is one. Because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should be. If both components in a solution are 50%, the term solute can be assigned to either component. How can you tell if a solution is saturated or unsaturated? Distinguish unsaturated, saturated, and supersaturated solutions.. Dilute Solution Or Unsaturated.
From www.youtube.com
Dilute solution,saturated solution, unsaturated solution,solubility Dilute Solution Or Unsaturated How can you tell if a solution is saturated or unsaturated? If both components in a solution are 50%, the term solute can be assigned to either component. A dilute solution is characterized by a low proportion of solute in the solvent, making it less concentrated. A solution that contains a relatively low concentration of solute is called dilute, and. Dilute Solution Or Unsaturated.
From www.slideserve.com
PPT What is osmosis? PowerPoint Presentation, free download ID2712925 Dilute Solution Or Unsaturated Distinguish unsaturated, saturated, and supersaturated solutions. A solution that contains a relatively low concentration of solute is called dilute, and one. The major component of the solution is called solvent, and the minor component (s) are called solute. If more solute is added and it does not dissolve, then the original solution was saturated. How can you tell if a. Dilute Solution Or Unsaturated.
From innobator.blogspot.com
INNOBATOR FÍSICA Y QUÍMICA 20192020 PHYSICS AND CHEMISTRY 2º ESO Dilute Solution Or Unsaturated A solution that contains a relatively low concentration of solute is called dilute, and one. Distinguish unsaturated, saturated, and supersaturated solutions. If the solute’s concentration is less than its solubility, the solution is said to be unsaturated. We will begin our discussion of solution concentration with two related and relative terms: A dilute solution is one. A dilute solution is. Dilute Solution Or Unsaturated.
From sciencenotes.org
Unsaturated Solution Definition and Examples in Chemistry Dilute Solution Or Unsaturated If more solute is added and it does not dissolve, then the original solution was saturated. A dilute solution is one. A solution that contains a relatively low concentration of solute is called dilute, and one. If both components in a solution are 50%, the term solute can be assigned to either component. We will begin our discussion of solution. Dilute Solution Or Unsaturated.
From slideplayer.com
Solutions. ppt download Dilute Solution Or Unsaturated Because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should be. We will begin our discussion of solution concentration with two related and relative terms: A dilute solution is one. Dilute solutions have a lower density and lighter color compared to concentrated solutions, while unsaturated solutions have the ability to.. Dilute Solution Or Unsaturated.
From slideplayer.com
Solutions!. ppt download Dilute Solution Or Unsaturated A dilute solution is characterized by a low proportion of solute in the solvent, making it less concentrated. How can you tell if a solution is saturated or unsaturated? A dilute solution is one. A solution that contains a relatively low concentration of solute is called dilute, and one. If more solute is added and it does not dissolve, then. Dilute Solution Or Unsaturated.
From slidetodoc.com
Saturated and Unsaturated Solutions Dissolving l l l Dilute Solution Or Unsaturated How can you tell if a solution is saturated or unsaturated? If both components in a solution are 50%, the term solute can be assigned to either component. Distinguish unsaturated, saturated, and supersaturated solutions. Dilute solutions have a lower density and lighter color compared to concentrated solutions, while unsaturated solutions have the ability to. The major component of the solution. Dilute Solution Or Unsaturated.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilute Solution Or Unsaturated A dilute solution is one. A solution that contains a relatively low concentration of solute is called dilute, and one. The major component of the solution is called solvent, and the minor component (s) are called solute. Because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should be. We will. Dilute Solution Or Unsaturated.
From slideplayer.com
(8th) Chapter 72 Cornell Notes ppt download Dilute Solution Or Unsaturated If both components in a solution are 50%, the term solute can be assigned to either component. The major component of the solution is called solvent, and the minor component (s) are called solute. A solution that contains a relatively low concentration of solute is called dilute, and one. Dilute solutions have a lower density and lighter color compared to. Dilute Solution Or Unsaturated.
From www.askiitians.com
Types Of Solutions Study Material for IIT JEE askIITians Dilute Solution Or Unsaturated Distinguish unsaturated, saturated, and supersaturated solutions. A dilute solution is one. The major component of the solution is called solvent, and the minor component (s) are called solute. Because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should be. We will begin our discussion of solution concentration with two related. Dilute Solution Or Unsaturated.
From www.youtube.com
SOLUTION DILUTE & CONC. SOLUTION SATURATED ,UNSATURATED & SUPER Dilute Solution Or Unsaturated If more solute is added and it does not dissolve, then the original solution was saturated. A dilute solution is characterized by a low proportion of solute in the solvent, making it less concentrated. Dilute solutions have a lower density and lighter color compared to concentrated solutions, while unsaturated solutions have the ability to. A dilute solution is one. A. Dilute Solution Or Unsaturated.
From www.teachoo.com
Classification of Acids on Basis of source, Concentration Teachoo Dilute Solution Or Unsaturated A dilute solution is characterized by a low proportion of solute in the solvent, making it less concentrated. A dilute solution is one. If the solute’s concentration is less than its solubility, the solution is said to be unsaturated. A solution that contains a relatively low concentration of solute is called dilute, and one. Dilute solutions have a lower density. Dilute Solution Or Unsaturated.
From slideplayer.com
Colligative Properties of Solutions ppt download Dilute Solution Or Unsaturated We will begin our discussion of solution concentration with two related and relative terms: A dilute solution is one. Dilute solutions have a lower density and lighter color compared to concentrated solutions, while unsaturated solutions have the ability to. How can you tell if a solution is saturated or unsaturated? A solution that contains a relatively low concentration of solute. Dilute Solution Or Unsaturated.