Heat Engine Adiabatic Expansion . there are several simple processes, used by heat engines, that flow from the first law of thermodynamics. No heat can transfer in or out of the system (q = 0). an adiabatic process is one in which no heat is gained or lost by the system. In this process, the system is thermally insulated. by definition, no heat is supplied in adiabatic expansion, but work is done. a reversible adiabatic gas expansion process. The gas continues to expand and do work on surroundings, which causes the system to cool to a lower temperature, \(t_{low}\). The work the gas does in adiabatic expansion is like that of a compressed. The first law of thermodynamics with q=0 shows that. an adiabatic process occurs when the system is isolated thermally. adiabatic expansion, the power stroke.
from www.numerade.com
adiabatic expansion, the power stroke. The first law of thermodynamics with q=0 shows that. there are several simple processes, used by heat engines, that flow from the first law of thermodynamics. by definition, no heat is supplied in adiabatic expansion, but work is done. a reversible adiabatic gas expansion process. The work the gas does in adiabatic expansion is like that of a compressed. The gas continues to expand and do work on surroundings, which causes the system to cool to a lower temperature, \(t_{low}\). an adiabatic process is one in which no heat is gained or lost by the system. No heat can transfer in or out of the system (q = 0). an adiabatic process occurs when the system is isolated thermally.
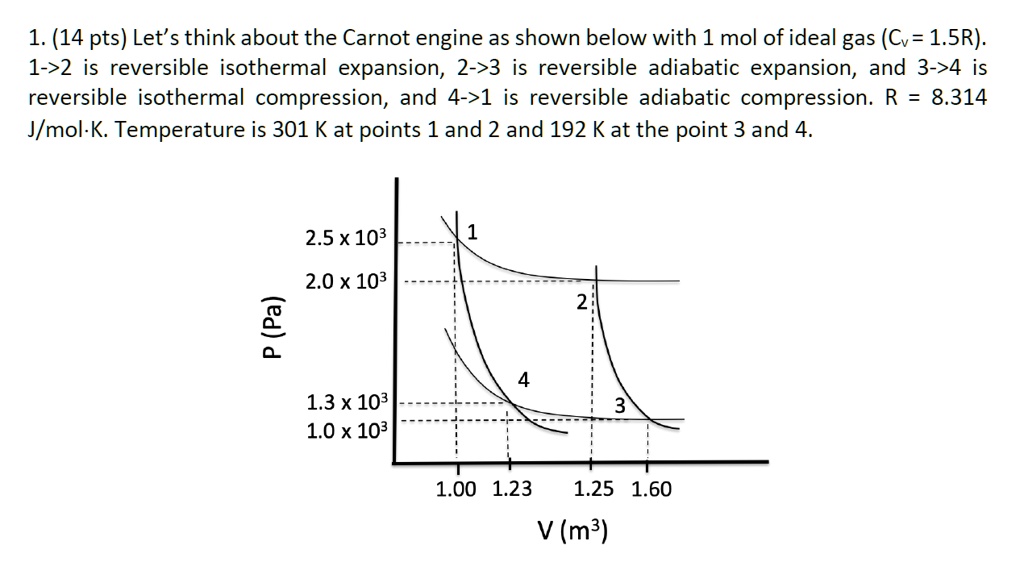
SOLVED 1. (14 pts) Let's think about the Carnot engine as shown below with 1 mol of ideal gas
Heat Engine Adiabatic Expansion there are several simple processes, used by heat engines, that flow from the first law of thermodynamics. No heat can transfer in or out of the system (q = 0). an adiabatic process occurs when the system is isolated thermally. The gas continues to expand and do work on surroundings, which causes the system to cool to a lower temperature, \(t_{low}\). In this process, the system is thermally insulated. The work the gas does in adiabatic expansion is like that of a compressed. an adiabatic process is one in which no heat is gained or lost by the system. a reversible adiabatic gas expansion process. The first law of thermodynamics with q=0 shows that. adiabatic expansion, the power stroke. there are several simple processes, used by heat engines, that flow from the first law of thermodynamics. by definition, no heat is supplied in adiabatic expansion, but work is done.
From www.numerade.com
SOLVED 1. (14 pts) Let's think about the Carnot engine as shown below with 1 mol of ideal gas Heat Engine Adiabatic Expansion an adiabatic process occurs when the system is isolated thermally. there are several simple processes, used by heat engines, that flow from the first law of thermodynamics. The first law of thermodynamics with q=0 shows that. The gas continues to expand and do work on surroundings, which causes the system to cool to a lower temperature, \(t_{low}\). . Heat Engine Adiabatic Expansion.
From wirelistetiquette.z13.web.core.windows.net
Carnot Cycle Ts Diagram Heat Engine Adiabatic Expansion The work the gas does in adiabatic expansion is like that of a compressed. adiabatic expansion, the power stroke. The gas continues to expand and do work on surroundings, which causes the system to cool to a lower temperature, \(t_{low}\). No heat can transfer in or out of the system (q = 0). an adiabatic process occurs when. Heat Engine Adiabatic Expansion.
From pressbooks.bccampus.ca
6.4 Carnot cycles Introduction to Engineering Thermodynamics Heat Engine Adiabatic Expansion there are several simple processes, used by heat engines, that flow from the first law of thermodynamics. The work the gas does in adiabatic expansion is like that of a compressed. adiabatic expansion, the power stroke. No heat can transfer in or out of the system (q = 0). an adiabatic process is one in which no. Heat Engine Adiabatic Expansion.
From www.numerade.com
SOLVEDThe figure below shows an engine cycle composed of a constant volume compression, an Heat Engine Adiabatic Expansion In this process, the system is thermally insulated. an adiabatic process occurs when the system is isolated thermally. The first law of thermodynamics with q=0 shows that. No heat can transfer in or out of the system (q = 0). a reversible adiabatic gas expansion process. by definition, no heat is supplied in adiabatic expansion, but work. Heat Engine Adiabatic Expansion.
From www.mechanicalbooster.com
What is Adiabatic Process Explanation Mechanical Booster Heat Engine Adiabatic Expansion No heat can transfer in or out of the system (q = 0). The work the gas does in adiabatic expansion is like that of a compressed. an adiabatic process is one in which no heat is gained or lost by the system. adiabatic expansion, the power stroke. a reversible adiabatic gas expansion process. an adiabatic. Heat Engine Adiabatic Expansion.
From brainly.in
An ideal gas used in Carnot engine has adiabatic expansion ratio 32. It’s specific heat ratio is Heat Engine Adiabatic Expansion The work the gas does in adiabatic expansion is like that of a compressed. adiabatic expansion, the power stroke. an adiabatic process is one in which no heat is gained or lost by the system. an adiabatic process occurs when the system is isolated thermally. No heat can transfer in or out of the system (q =. Heat Engine Adiabatic Expansion.
From www.google.com
Patent US8156739 Adiabatic expansion heat engine and method of operating Google Patents Heat Engine Adiabatic Expansion In this process, the system is thermally insulated. The gas continues to expand and do work on surroundings, which causes the system to cool to a lower temperature, \(t_{low}\). adiabatic expansion, the power stroke. by definition, no heat is supplied in adiabatic expansion, but work is done. there are several simple processes, used by heat engines, that. Heat Engine Adiabatic Expansion.
From www.doubtnut.com
The efficiency of a heat engine is defined as the ratio of the mechanical work done by the Heat Engine Adiabatic Expansion The gas continues to expand and do work on surroundings, which causes the system to cool to a lower temperature, \(t_{low}\). The work the gas does in adiabatic expansion is like that of a compressed. there are several simple processes, used by heat engines, that flow from the first law of thermodynamics. No heat can transfer in or out. Heat Engine Adiabatic Expansion.
From wireblueprint.com
Exploring the Adiabatic Expansion Process with PV Diagrams Heat Engine Adiabatic Expansion No heat can transfer in or out of the system (q = 0). a reversible adiabatic gas expansion process. The first law of thermodynamics with q=0 shows that. an adiabatic process is one in which no heat is gained or lost by the system. there are several simple processes, used by heat engines, that flow from the. Heat Engine Adiabatic Expansion.
From www.brainkart.com
Heat Engine Thermodynamics Heat Engine Adiabatic Expansion by definition, no heat is supplied in adiabatic expansion, but work is done. No heat can transfer in or out of the system (q = 0). The gas continues to expand and do work on surroundings, which causes the system to cool to a lower temperature, \(t_{low}\). there are several simple processes, used by heat engines, that flow. Heat Engine Adiabatic Expansion.
From adpokat.github.io
What Is Adiabatic Process Heat Engine Adiabatic Expansion The gas continues to expand and do work on surroundings, which causes the system to cool to a lower temperature, \(t_{low}\). an adiabatic process is one in which no heat is gained or lost by the system. there are several simple processes, used by heat engines, that flow from the first law of thermodynamics. an adiabatic process. Heat Engine Adiabatic Expansion.
From www.reddit.com
I would like to make an interactive PV diagram (thermodynamics) however I cannot break it Heat Engine Adiabatic Expansion The gas continues to expand and do work on surroundings, which causes the system to cool to a lower temperature, \(t_{low}\). by definition, no heat is supplied in adiabatic expansion, but work is done. there are several simple processes, used by heat engines, that flow from the first law of thermodynamics. No heat can transfer in or out. Heat Engine Adiabatic Expansion.
From www.numerade.com
SOLVED 5.2 THE CARNOT CYCLE The operation of an arbitrary heat engine is represented in Fig 5 Heat Engine Adiabatic Expansion No heat can transfer in or out of the system (q = 0). In this process, the system is thermally insulated. The gas continues to expand and do work on surroundings, which causes the system to cool to a lower temperature, \(t_{low}\). a reversible adiabatic gas expansion process. an adiabatic process is one in which no heat is. Heat Engine Adiabatic Expansion.
From www.youtube.com
Thermodynamics reversible adiabatic expansion, colorcoded derivation YouTube Heat Engine Adiabatic Expansion an adiabatic process is one in which no heat is gained or lost by the system. The gas continues to expand and do work on surroundings, which causes the system to cool to a lower temperature, \(t_{low}\). The first law of thermodynamics with q=0 shows that. an adiabatic process occurs when the system is isolated thermally. adiabatic. Heat Engine Adiabatic Expansion.
From www.eigenplus.com
carnot_cycle_diagram eigenplus Heat Engine Adiabatic Expansion The work the gas does in adiabatic expansion is like that of a compressed. there are several simple processes, used by heat engines, that flow from the first law of thermodynamics. a reversible adiabatic gas expansion process. an adiabatic process occurs when the system is isolated thermally. In this process, the system is thermally insulated. The gas. Heat Engine Adiabatic Expansion.
From circuitdbsirenize.z13.web.core.windows.net
Pv Diagram For Adiabatic Process Heat Engine Adiabatic Expansion The work the gas does in adiabatic expansion is like that of a compressed. adiabatic expansion, the power stroke. The first law of thermodynamics with q=0 shows that. an adiabatic process occurs when the system is isolated thermally. In this process, the system is thermally insulated. The gas continues to expand and do work on surroundings, which causes. Heat Engine Adiabatic Expansion.
From kenkidryer.com
Adiabatic expansion and adiabatic compression KENKI DRYER Heat Engine Adiabatic Expansion In this process, the system is thermally insulated. adiabatic expansion, the power stroke. The gas continues to expand and do work on surroundings, which causes the system to cool to a lower temperature, \(t_{low}\). The work the gas does in adiabatic expansion is like that of a compressed. No heat can transfer in or out of the system (q. Heat Engine Adiabatic Expansion.
From www.tec-science.com
Thermodynamic systems tecscience Heat Engine Adiabatic Expansion a reversible adiabatic gas expansion process. there are several simple processes, used by heat engines, that flow from the first law of thermodynamics. In this process, the system is thermally insulated. The first law of thermodynamics with q=0 shows that. by definition, no heat is supplied in adiabatic expansion, but work is done. an adiabatic process. Heat Engine Adiabatic Expansion.
From www.chegg.com
Solved Isothermal expansion at temperature T Adiabatic Heat Engine Adiabatic Expansion a reversible adiabatic gas expansion process. by definition, no heat is supplied in adiabatic expansion, but work is done. In this process, the system is thermally insulated. The work the gas does in adiabatic expansion is like that of a compressed. an adiabatic process occurs when the system is isolated thermally. The gas continues to expand and. Heat Engine Adiabatic Expansion.
From www.youtube.com
Thermodynamic Processes Isobaric, Isochoric, Isothermal and Adiabatic process Chemistry 12 Heat Engine Adiabatic Expansion No heat can transfer in or out of the system (q = 0). a reversible adiabatic gas expansion process. there are several simple processes, used by heat engines, that flow from the first law of thermodynamics. an adiabatic process is one in which no heat is gained or lost by the system. The work the gas does. Heat Engine Adiabatic Expansion.
From www.slideserve.com
PPT The Adiabatic Expansion of an Ideal Gas PowerPoint Presentation, free download ID3187786 Heat Engine Adiabatic Expansion an adiabatic process occurs when the system is isolated thermally. In this process, the system is thermally insulated. a reversible adiabatic gas expansion process. adiabatic expansion, the power stroke. by definition, no heat is supplied in adiabatic expansion, but work is done. The gas continues to expand and do work on surroundings, which causes the system. Heat Engine Adiabatic Expansion.
From www.physicsforums.com
Adiabatic Process in a heat engine Heat Engine Adiabatic Expansion The first law of thermodynamics with q=0 shows that. an adiabatic process occurs when the system is isolated thermally. In this process, the system is thermally insulated. adiabatic expansion, the power stroke. No heat can transfer in or out of the system (q = 0). The gas continues to expand and do work on surroundings, which causes the. Heat Engine Adiabatic Expansion.
From readchemistry.com
Adiabatic Expansion of an Ideal Gas Read Chemistry Heat Engine Adiabatic Expansion by definition, no heat is supplied in adiabatic expansion, but work is done. The gas continues to expand and do work on surroundings, which causes the system to cool to a lower temperature, \(t_{low}\). a reversible adiabatic gas expansion process. The first law of thermodynamics with q=0 shows that. No heat can transfer in or out of the. Heat Engine Adiabatic Expansion.
From collegedunia.com
Adiabatic Process Derivation Formula, Examples & Equation Heat Engine Adiabatic Expansion an adiabatic process is one in which no heat is gained or lost by the system. The work the gas does in adiabatic expansion is like that of a compressed. an adiabatic process occurs when the system is isolated thermally. there are several simple processes, used by heat engines, that flow from the first law of thermodynamics.. Heat Engine Adiabatic Expansion.
From en.wikipedia.org
Adiabatic process Wikipedia Heat Engine Adiabatic Expansion an adiabatic process is one in which no heat is gained or lost by the system. The work the gas does in adiabatic expansion is like that of a compressed. an adiabatic process occurs when the system is isolated thermally. a reversible adiabatic gas expansion process. In this process, the system is thermally insulated. No heat can. Heat Engine Adiabatic Expansion.
From circuitdbclicheed.z13.web.core.windows.net
Pv Diagram Thermodynamics Heat Engine Adiabatic Expansion by definition, no heat is supplied in adiabatic expansion, but work is done. an adiabatic process is one in which no heat is gained or lost by the system. adiabatic expansion, the power stroke. The work the gas does in adiabatic expansion is like that of a compressed. The first law of thermodynamics with q=0 shows that.. Heat Engine Adiabatic Expansion.
From www.slideserve.com
PPT Adiabatic Processes PowerPoint Presentation, free download ID5766036 Heat Engine Adiabatic Expansion an adiabatic process is one in which no heat is gained or lost by the system. an adiabatic process occurs when the system is isolated thermally. there are several simple processes, used by heat engines, that flow from the first law of thermodynamics. The work the gas does in adiabatic expansion is like that of a compressed.. Heat Engine Adiabatic Expansion.
From www.google.com
Patent US8156739 Adiabatic expansion heat engine and method of operating Google Patents Heat Engine Adiabatic Expansion there are several simple processes, used by heat engines, that flow from the first law of thermodynamics. an adiabatic process is one in which no heat is gained or lost by the system. adiabatic expansion, the power stroke. a reversible adiabatic gas expansion process. an adiabatic process occurs when the system is isolated thermally. The. Heat Engine Adiabatic Expansion.
From www.youtube.com
Adiabatic expansion of an ideal gas YouTube Heat Engine Adiabatic Expansion adiabatic expansion, the power stroke. a reversible adiabatic gas expansion process. there are several simple processes, used by heat engines, that flow from the first law of thermodynamics. by definition, no heat is supplied in adiabatic expansion, but work is done. The gas continues to expand and do work on surroundings, which causes the system to. Heat Engine Adiabatic Expansion.
From www.youtube.com
Adiabatic Expansion of Steam YouTube Heat Engine Adiabatic Expansion The work the gas does in adiabatic expansion is like that of a compressed. an adiabatic process is one in which no heat is gained or lost by the system. a reversible adiabatic gas expansion process. In this process, the system is thermally insulated. an adiabatic process occurs when the system is isolated thermally. by definition,. Heat Engine Adiabatic Expansion.
From pressbooks.bccampus.ca
15.3 Introduction to the Second Law of Thermodynamics Heat Engines and Their Efficiency Heat Engine Adiabatic Expansion by definition, no heat is supplied in adiabatic expansion, but work is done. adiabatic expansion, the power stroke. an adiabatic process occurs when the system is isolated thermally. a reversible adiabatic gas expansion process. In this process, the system is thermally insulated. there are several simple processes, used by heat engines, that flow from the. Heat Engine Adiabatic Expansion.
From philschatz.com
Applications of Thermodynamics Heat Pumps and Refrigerators · Physics Heat Engine Adiabatic Expansion a reversible adiabatic gas expansion process. The gas continues to expand and do work on surroundings, which causes the system to cool to a lower temperature, \(t_{low}\). an adiabatic process occurs when the system is isolated thermally. an adiabatic process is one in which no heat is gained or lost by the system. The first law of. Heat Engine Adiabatic Expansion.
From www.numerade.com
SOLVED Consider a heat engine operating on n moles of an ideal gas. The engine cycle consists Heat Engine Adiabatic Expansion an adiabatic process occurs when the system is isolated thermally. The first law of thermodynamics with q=0 shows that. The gas continues to expand and do work on surroundings, which causes the system to cool to a lower temperature, \(t_{low}\). there are several simple processes, used by heat engines, that flow from the first law of thermodynamics. The. Heat Engine Adiabatic Expansion.
From 88guru.com
Adiabatic Process Definition, Equation, Reversible 88Guru Heat Engine Adiabatic Expansion a reversible adiabatic gas expansion process. The gas continues to expand and do work on surroundings, which causes the system to cool to a lower temperature, \(t_{low}\). an adiabatic process occurs when the system is isolated thermally. an adiabatic process is one in which no heat is gained or lost by the system. by definition, no. Heat Engine Adiabatic Expansion.
From www.youtube.com
Adiabatic Process Work, Heat & Internal Energy, Gamma Ratio, Thermodynamics & Physics YouTube Heat Engine Adiabatic Expansion there are several simple processes, used by heat engines, that flow from the first law of thermodynamics. In this process, the system is thermally insulated. by definition, no heat is supplied in adiabatic expansion, but work is done. The work the gas does in adiabatic expansion is like that of a compressed. No heat can transfer in or. Heat Engine Adiabatic Expansion.