What Is The Outer Shell Electron Configuration In Group 1A . the outer electron gets further from the nucleus; group 1a (ia) elements have one electron. Group 2a (iia) elements have two electrons. within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1 (group 13). Atomic structure and electron configuration. how is an electron's outer electron configuration related to its position in the periodic table? What are unabbreviated and abbreviated electron. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. The periodic table, electron shells, and orbitals. this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of. What about a redox reaction?
from general.chemistrysteps.com
within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1 (group 13). Group 2a (iia) elements have two electrons. What about a redox reaction? the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of. Atomic structure and electron configuration. the outer electron gets further from the nucleus; group 1a (ia) elements have one electron. how is an electron's outer electron configuration related to its position in the periodic table? The periodic table, electron shells, and orbitals.
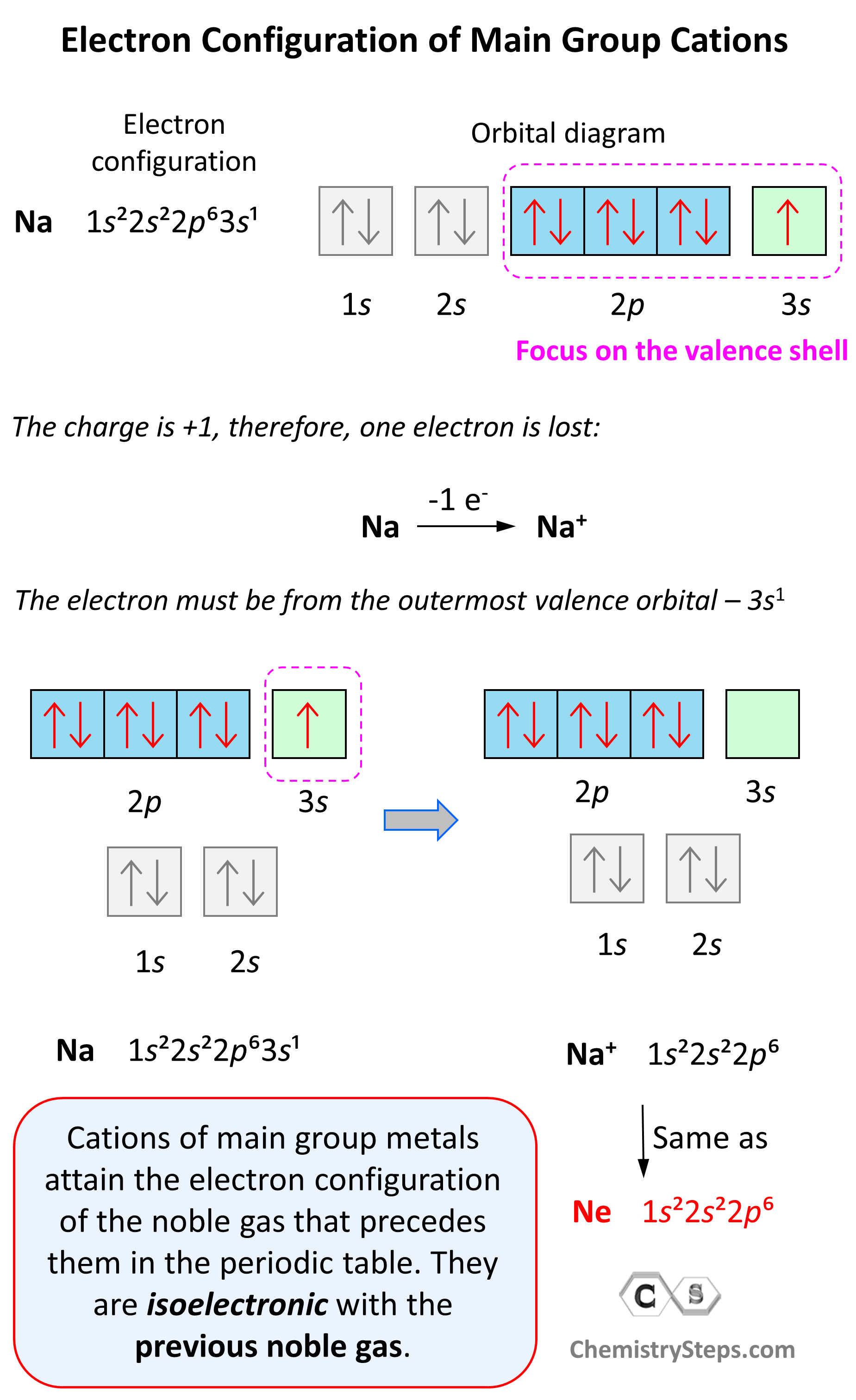
Electron Configurations of Ions Chemistry Steps
What Is The Outer Shell Electron Configuration In Group 1A the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. The periodic table, electron shells, and orbitals. Group 2a (iia) elements have two electrons. group 1a (ia) elements have one electron. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. What are unabbreviated and abbreviated electron. What about a redox reaction? within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1 (group 13). how is an electron's outer electron configuration related to its position in the periodic table? this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of. the outer electron gets further from the nucleus; Atomic structure and electron configuration.
From www.goodscience.com.au
Electron Configuration (Elements 120) Good Science What Is The Outer Shell Electron Configuration In Group 1A What are unabbreviated and abbreviated electron. how is an electron's outer electron configuration related to its position in the periodic table? The periodic table, electron shells, and orbitals. this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of. What about a redox reaction? the electron configurations of silicon (14 electrons), phosphorus. What Is The Outer Shell Electron Configuration In Group 1A.
From keystagewiki.com
Electron Orbital Key Stage Wiki What Is The Outer Shell Electron Configuration In Group 1A how is an electron's outer electron configuration related to its position in the periodic table? What about a redox reaction? within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1 (group 13). Group 2a (iia) elements have two electrons. Atomic structure and electron configuration. the outer. What Is The Outer Shell Electron Configuration In Group 1A.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry What Is The Outer Shell Electron Configuration In Group 1A group 1a (ia) elements have one electron. how is an electron's outer electron configuration related to its position in the periodic table? What about a redox reaction? Group 2a (iia) elements have two electrons. What are unabbreviated and abbreviated electron. this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of. Atomic. What Is The Outer Shell Electron Configuration In Group 1A.
From sciencenotes.org
Periodic Table Showing Shells What Is The Outer Shell Electron Configuration In Group 1A Group 2a (iia) elements have two electrons. What are unabbreviated and abbreviated electron. Atomic structure and electron configuration. The periodic table, electron shells, and orbitals. What about a redox reaction? within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1 (group 13). the electron configurations of silicon. What Is The Outer Shell Electron Configuration In Group 1A.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics What Is The Outer Shell Electron Configuration In Group 1A What about a redox reaction? the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. Group 2a (iia) elements have two electrons. The periodic table, electron shells, and orbitals. the outer electron gets further from the nucleus; What are unabbreviated and abbreviated electron. Atomic structure and electron configuration. this electron. What Is The Outer Shell Electron Configuration In Group 1A.
From www.allaboutcircuits.com
Valence and Crystal Structure Solidstate Device Theory Electronics What Is The Outer Shell Electron Configuration In Group 1A how is an electron's outer electron configuration related to its position in the periodic table? The periodic table, electron shells, and orbitals. Atomic structure and electron configuration. What are unabbreviated and abbreviated electron. Group 2a (iia) elements have two electrons. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. What. What Is The Outer Shell Electron Configuration In Group 1A.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps What Is The Outer Shell Electron Configuration In Group 1A the outer electron gets further from the nucleus; how is an electron's outer electron configuration related to its position in the periodic table? group 1a (ia) elements have one electron. The periodic table, electron shells, and orbitals. Group 2a (iia) elements have two electrons. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16. What Is The Outer Shell Electron Configuration In Group 1A.
From www.thoughtco.com
Electron Configuration Chart What Is The Outer Shell Electron Configuration In Group 1A how is an electron's outer electron configuration related to its position in the periodic table? group 1a (ia) elements have one electron. What are unabbreviated and abbreviated electron. Atomic structure and electron configuration. What about a redox reaction? the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. Group 2a. What Is The Outer Shell Electron Configuration In Group 1A.
From sciencenotes.org
Periodic Table Outermost Electron Orbitals What Is The Outer Shell Electron Configuration In Group 1A how is an electron's outer electron configuration related to its position in the periodic table? What about a redox reaction? Group 2a (iia) elements have two electrons. What are unabbreviated and abbreviated electron. this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of. the outer electron gets further from the nucleus;. What Is The Outer Shell Electron Configuration In Group 1A.
From www.online-sciences.com
Elements of sblock, Properties of the first group elements 1A (Alkali What Is The Outer Shell Electron Configuration In Group 1A this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of. Group 2a (iia) elements have two electrons. Atomic structure and electron configuration. within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1 (group 13). the outer electron gets further from. What Is The Outer Shell Electron Configuration In Group 1A.
From www.technologyuk.net
Chemical Bonding What Is The Outer Shell Electron Configuration In Group 1A group 1a (ia) elements have one electron. this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of. Atomic structure and electron configuration. within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1 (group 13). how is an electron's outer. What Is The Outer Shell Electron Configuration In Group 1A.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram What Is The Outer Shell Electron Configuration In Group 1A within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1 (group 13). group 1a (ia) elements have one electron. Group 2a (iia) elements have two electrons. how is an electron's outer electron configuration related to its position in the periodic table? the outer electron gets. What Is The Outer Shell Electron Configuration In Group 1A.
From www.vectorstock.com
Periodic table element showing electron shells Vector Image What Is The Outer Shell Electron Configuration In Group 1A What about a redox reaction? Group 2a (iia) elements have two electrons. Atomic structure and electron configuration. group 1a (ia) elements have one electron. how is an electron's outer electron configuration related to its position in the periodic table? the outer electron gets further from the nucleus; this electron configuration calculator will instantly show you the. What Is The Outer Shell Electron Configuration In Group 1A.
From exooogfvb.blob.core.windows.net
What Is The Electron Distribution In Shells at Ruby Lehmann blog What Is The Outer Shell Electron Configuration In Group 1A Group 2a (iia) elements have two electrons. the outer electron gets further from the nucleus; What are unabbreviated and abbreviated electron. this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of. Atomic structure and electron configuration. group 1a (ia) elements have one electron. the electron configurations of silicon (14 electrons),. What Is The Outer Shell Electron Configuration In Group 1A.
From sciencenotes.org
List of Electron Configurations of Elements What Is The Outer Shell Electron Configuration In Group 1A this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of. how is an electron's outer electron configuration related to its position in the periodic table? within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1 (group 13). What are unabbreviated. What Is The Outer Shell Electron Configuration In Group 1A.
From philschatz.com
Electronic Structure of Atoms (Electron Configurations) · Chemistry What Is The Outer Shell Electron Configuration In Group 1A The periodic table, electron shells, and orbitals. group 1a (ia) elements have one electron. What about a redox reaction? What are unabbreviated and abbreviated electron. this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of. Atomic structure and electron configuration. how is an electron's outer electron configuration related to its position. What Is The Outer Shell Electron Configuration In Group 1A.
From www.animalia-life.club
Electron Shell Diagram What Is The Outer Shell Electron Configuration In Group 1A the outer electron gets further from the nucleus; What are unabbreviated and abbreviated electron. What about a redox reaction? Group 2a (iia) elements have two electrons. Atomic structure and electron configuration. The periodic table, electron shells, and orbitals. this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of. within each column,. What Is The Outer Shell Electron Configuration In Group 1A.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements What Is The Outer Shell Electron Configuration In Group 1A this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of. Group 2a (iia) elements have two electrons. What are unabbreviated and abbreviated electron. group 1a (ia) elements have one electron. the outer electron gets further from the nucleus; The periodic table, electron shells, and orbitals. Atomic structure and electron configuration. What. What Is The Outer Shell Electron Configuration In Group 1A.
From acemichael888.weebly.com
Electron Arrangement in Atoms Elements and the Periodic Table What Is The Outer Shell Electron Configuration In Group 1A how is an electron's outer electron configuration related to its position in the periodic table? within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1 (group 13). Group 2a (iia) elements have two electrons. this electron configuration calculator will instantly show you the distribution of electrons. What Is The Outer Shell Electron Configuration In Group 1A.
From exovyidbs.blob.core.windows.net
How To Find Electron Shells In An Element at Jonathan Brown blog What Is The Outer Shell Electron Configuration In Group 1A Atomic structure and electron configuration. What are unabbreviated and abbreviated electron. What about a redox reaction? the outer electron gets further from the nucleus; The periodic table, electron shells, and orbitals. Group 2a (iia) elements have two electrons. within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np. What Is The Outer Shell Electron Configuration In Group 1A.
From mungfali.com
What Is An Electron Shell What Is The Outer Shell Electron Configuration In Group 1A within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1 (group 13). The periodic table, electron shells, and orbitals. group 1a (ia) elements have one electron. Group 2a (iia) elements have two electrons. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons),. What Is The Outer Shell Electron Configuration In Group 1A.
From www.britannica.com
Electron shell Definition & Facts Britannica What Is The Outer Shell Electron Configuration In Group 1A What about a redox reaction? the outer electron gets further from the nucleus; What are unabbreviated and abbreviated electron. this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of. The periodic table, electron shells, and orbitals. Atomic structure and electron configuration. how is an electron's outer electron configuration related to its. What Is The Outer Shell Electron Configuration In Group 1A.
From chemistry291.blogspot.com
What Is the Electron Configuration with Step by Step Guides to write What Is The Outer Shell Electron Configuration In Group 1A Atomic structure and electron configuration. how is an electron's outer electron configuration related to its position in the periodic table? What are unabbreviated and abbreviated electron. the outer electron gets further from the nucleus; this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of. group 1a (ia) elements have one. What Is The Outer Shell Electron Configuration In Group 1A.
From www.numerade.com
SOLVEDAnswer true or false. (a) Elements in the same column of the What Is The Outer Shell Electron Configuration In Group 1A the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. Group 2a (iia) elements have two electrons. within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1 (group 13). group 1a (ia) elements have one electron. The periodic table,. What Is The Outer Shell Electron Configuration In Group 1A.
From www.expii.com
Valence Electrons — Definition & Importance Expii What Is The Outer Shell Electron Configuration In Group 1A the outer electron gets further from the nucleus; The periodic table, electron shells, and orbitals. within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1 (group 13). the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. this. What Is The Outer Shell Electron Configuration In Group 1A.
From www.dynamicscience.com.au
chemistry periodic table and electron configuration What Is The Outer Shell Electron Configuration In Group 1A Group 2a (iia) elements have two electrons. within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1 (group 13). this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of. group 1a (ia) elements have one electron. the electron configurations. What Is The Outer Shell Electron Configuration In Group 1A.
From chemwiki.ucdavis.edu
Electronic Configurations Chemwiki What Is The Outer Shell Electron Configuration In Group 1A how is an electron's outer electron configuration related to its position in the periodic table? What are unabbreviated and abbreviated electron. group 1a (ia) elements have one electron. Atomic structure and electron configuration. the outer electron gets further from the nucleus; What about a redox reaction? within each column, each element has the same valence electron. What Is The Outer Shell Electron Configuration In Group 1A.
From cronodon.com
Introduction to Atoms What Is The Outer Shell Electron Configuration In Group 1A What are unabbreviated and abbreviated electron. this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of. group 1a (ia) elements have one electron. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. Atomic structure and electron configuration. The periodic table, electron shells, and. What Is The Outer Shell Electron Configuration In Group 1A.
From courses.lumenlearning.com
Electronic Structure of Atoms (Electron Configurations) Chemistry What Is The Outer Shell Electron Configuration In Group 1A the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. What about a redox reaction? within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1 (group 13). this electron configuration calculator will instantly show you the distribution of electrons. What Is The Outer Shell Electron Configuration In Group 1A.
From www.breakingatom.com
Group 1 The Alkali Metals What Is The Outer Shell Electron Configuration In Group 1A group 1a (ia) elements have one electron. how is an electron's outer electron configuration related to its position in the periodic table? Group 2a (iia) elements have two electrons. the outer electron gets further from the nucleus; What about a redox reaction? What are unabbreviated and abbreviated electron. this electron configuration calculator will instantly show you. What Is The Outer Shell Electron Configuration In Group 1A.
From www.sciencefacts.net
Electron Shell Definition & Number of Electrons in Each Shell What Is The Outer Shell Electron Configuration In Group 1A Atomic structure and electron configuration. The periodic table, electron shells, and orbitals. within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1 (group 13). group 1a (ia) elements have one electron. how is an electron's outer electron configuration related to its position in the periodic table?. What Is The Outer Shell Electron Configuration In Group 1A.
From homedeso.vercel.app
Group 1 Periodic Table Electron Configuration What Is The Outer Shell Electron Configuration In Group 1A how is an electron's outer electron configuration related to its position in the periodic table? the outer electron gets further from the nucleus; What are unabbreviated and abbreviated electron. Group 2a (iia) elements have two electrons. group 1a (ia) elements have one electron. The periodic table, electron shells, and orbitals. within each column, each element has. What Is The Outer Shell Electron Configuration In Group 1A.
From commons.wikimedia.org
FilePeriodic table of elements showing electron shells.png What Is The Outer Shell Electron Configuration In Group 1A group 1a (ia) elements have one electron. Group 2a (iia) elements have two electrons. how is an electron's outer electron configuration related to its position in the periodic table? the outer electron gets further from the nucleus; the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. What are. What Is The Outer Shell Electron Configuration In Group 1A.
From chem.libretexts.org
6.4 Electronic Structure of Atoms (Electron Configurations What Is The Outer Shell Electron Configuration In Group 1A the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. how is an electron's outer electron configuration related to its position in the periodic table? The periodic table, electron shells, and orbitals. this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of. Group 2a. What Is The Outer Shell Electron Configuration In Group 1A.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table What Is The Outer Shell Electron Configuration In Group 1A the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. how is an electron's outer electron configuration related to its position in the periodic table? What about a redox reaction? the outer electron gets further from the nucleus; Group 2a (iia) elements have two electrons. What are unabbreviated and abbreviated. What Is The Outer Shell Electron Configuration In Group 1A.