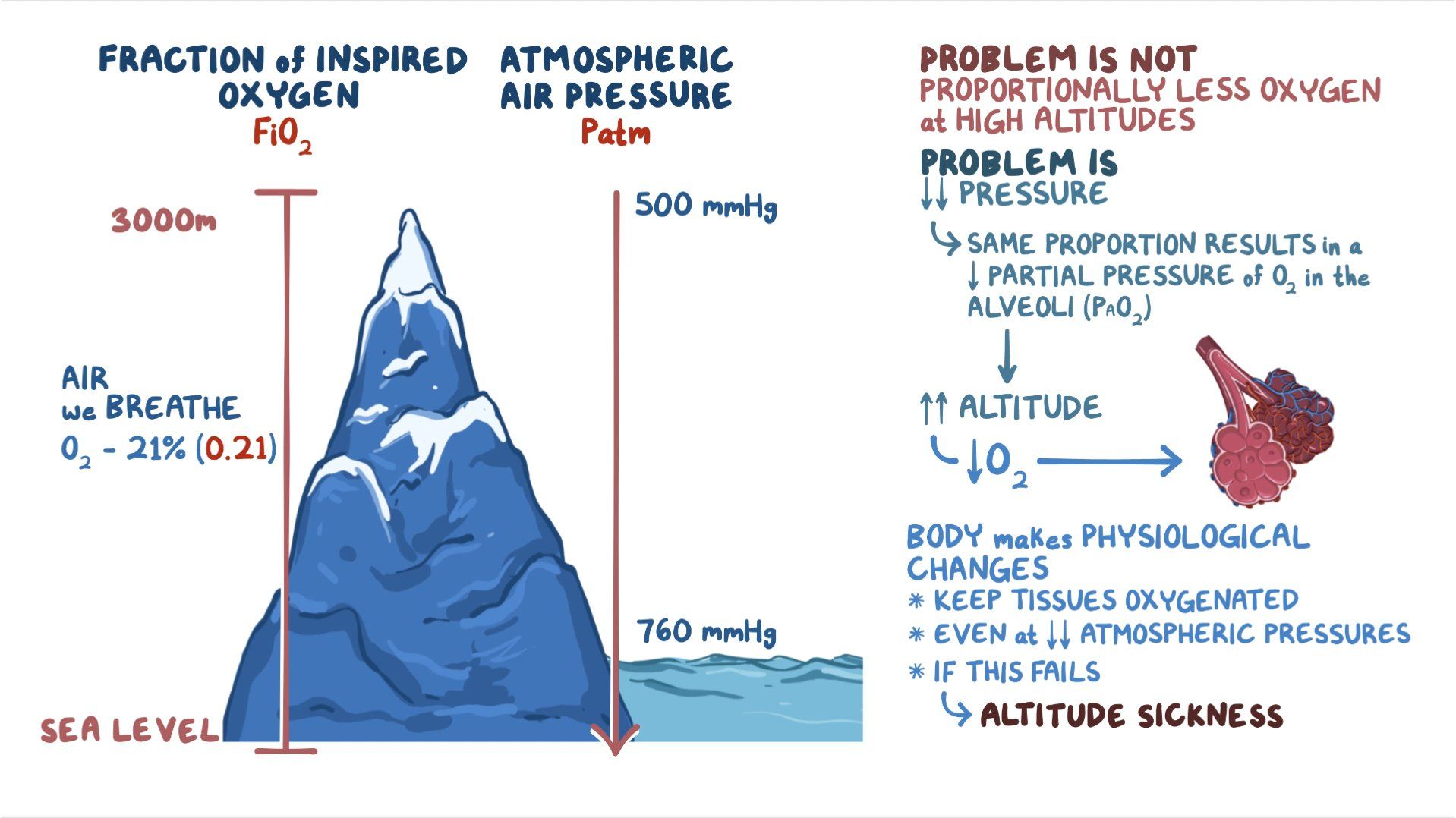
Barometric Pressure And Oxygen Levels . a drop in atmospheric pressure, as observed at high altitudes, leads to decreased oxygen saturation. The air around you has weight, and it presses against everything it touches. above sea level, the declines in o 2 partial pressure will be smaller than at. The effect of regular changes. hyperbaric oxygen therapy utilizes the virtue of increased oxygen levels at an elevated atmospheric pressure. the chart is based on the ideal gas law equation for pressure versus altitude (barometric formula), assuming a constant atmospheric temperature. That pressure is called atmospheric. at sea level, during normal conditions, the partial pressure of oxygen in the arteries is high enough to satisfy the.
from www.osmosis.org
at sea level, during normal conditions, the partial pressure of oxygen in the arteries is high enough to satisfy the. That pressure is called atmospheric. the chart is based on the ideal gas law equation for pressure versus altitude (barometric formula), assuming a constant atmospheric temperature. The air around you has weight, and it presses against everything it touches. The effect of regular changes. hyperbaric oxygen therapy utilizes the virtue of increased oxygen levels at an elevated atmospheric pressure. above sea level, the declines in o 2 partial pressure will be smaller than at. a drop in atmospheric pressure, as observed at high altitudes, leads to decreased oxygen saturation.
Pulmonary changes at high altitude and altitude sickness Osmosis
Barometric Pressure And Oxygen Levels the chart is based on the ideal gas law equation for pressure versus altitude (barometric formula), assuming a constant atmospheric temperature. The air around you has weight, and it presses against everything it touches. above sea level, the declines in o 2 partial pressure will be smaller than at. That pressure is called atmospheric. the chart is based on the ideal gas law equation for pressure versus altitude (barometric formula), assuming a constant atmospheric temperature. at sea level, during normal conditions, the partial pressure of oxygen in the arteries is high enough to satisfy the. a drop in atmospheric pressure, as observed at high altitudes, leads to decreased oxygen saturation. The effect of regular changes. hyperbaric oxygen therapy utilizes the virtue of increased oxygen levels at an elevated atmospheric pressure.
From breathe.ersjournals.com
Relating oxygen partial pressure, saturation and content the Barometric Pressure And Oxygen Levels a drop in atmospheric pressure, as observed at high altitudes, leads to decreased oxygen saturation. The effect of regular changes. the chart is based on the ideal gas law equation for pressure versus altitude (barometric formula), assuming a constant atmospheric temperature. The air around you has weight, and it presses against everything it touches. above sea level,. Barometric Pressure And Oxygen Levels.
From www.circuitbasics.com
How to Set Up the BMP180 Barometric Pressure Sensor on an Arduino Barometric Pressure And Oxygen Levels a drop in atmospheric pressure, as observed at high altitudes, leads to decreased oxygen saturation. above sea level, the declines in o 2 partial pressure will be smaller than at. That pressure is called atmospheric. the chart is based on the ideal gas law equation for pressure versus altitude (barometric formula), assuming a constant atmospheric temperature. The. Barometric Pressure And Oxygen Levels.
From www.researchgate.net
Pressures and oxygen concentrations at altitude 24 Download Barometric Pressure And Oxygen Levels The effect of regular changes. a drop in atmospheric pressure, as observed at high altitudes, leads to decreased oxygen saturation. the chart is based on the ideal gas law equation for pressure versus altitude (barometric formula), assuming a constant atmospheric temperature. above sea level, the declines in o 2 partial pressure will be smaller than at. . Barometric Pressure And Oxygen Levels.
From thoracickey.com
High altitude and flying Thoracic Key Barometric Pressure And Oxygen Levels the chart is based on the ideal gas law equation for pressure versus altitude (barometric formula), assuming a constant atmospheric temperature. That pressure is called atmospheric. at sea level, during normal conditions, the partial pressure of oxygen in the arteries is high enough to satisfy the. The effect of regular changes. The air around you has weight, and. Barometric Pressure And Oxygen Levels.
From www.researchgate.net
Relationship between elevation and Barometric Pressure (filled circles Barometric Pressure And Oxygen Levels The effect of regular changes. The air around you has weight, and it presses against everything it touches. That pressure is called atmospheric. hyperbaric oxygen therapy utilizes the virtue of increased oxygen levels at an elevated atmospheric pressure. the chart is based on the ideal gas law equation for pressure versus altitude (barometric formula), assuming a constant atmospheric. Barometric Pressure And Oxygen Levels.
From derangedphysiology.com
The oxygen cascade Deranged Physiology Barometric Pressure And Oxygen Levels The air around you has weight, and it presses against everything it touches. the chart is based on the ideal gas law equation for pressure versus altitude (barometric formula), assuming a constant atmospheric temperature. a drop in atmospheric pressure, as observed at high altitudes, leads to decreased oxygen saturation. That pressure is called atmospheric. hyperbaric oxygen therapy. Barometric Pressure And Oxygen Levels.
From bmj.com
Oxygen at high altitude The BMJ Barometric Pressure And Oxygen Levels hyperbaric oxygen therapy utilizes the virtue of increased oxygen levels at an elevated atmospheric pressure. above sea level, the declines in o 2 partial pressure will be smaller than at. The air around you has weight, and it presses against everything it touches. The effect of regular changes. That pressure is called atmospheric. at sea level, during. Barometric Pressure And Oxygen Levels.
From inspiritvr.com
Air pressure at Mount Everest Study Guide Inspirit Learning Inc Barometric Pressure And Oxygen Levels That pressure is called atmospheric. The effect of regular changes. above sea level, the declines in o 2 partial pressure will be smaller than at. the chart is based on the ideal gas law equation for pressure versus altitude (barometric formula), assuming a constant atmospheric temperature. hyperbaric oxygen therapy utilizes the virtue of increased oxygen levels at. Barometric Pressure And Oxygen Levels.
From mungfali.com
Oxygen Levels Altitude Chart Barometric Pressure And Oxygen Levels at sea level, during normal conditions, the partial pressure of oxygen in the arteries is high enough to satisfy the. The air around you has weight, and it presses against everything it touches. a drop in atmospheric pressure, as observed at high altitudes, leads to decreased oxygen saturation. The effect of regular changes. above sea level, the. Barometric Pressure And Oxygen Levels.
From www.pinterest.de
Pin on Diagrams and Infographics Barometric Pressure And Oxygen Levels The air around you has weight, and it presses against everything it touches. That pressure is called atmospheric. above sea level, the declines in o 2 partial pressure will be smaller than at. a drop in atmospheric pressure, as observed at high altitudes, leads to decreased oxygen saturation. The effect of regular changes. hyperbaric oxygen therapy utilizes. Barometric Pressure And Oxygen Levels.
From www.medintensiva.org
A journey between high altitude hypoxia and critical patient hypoxia Barometric Pressure And Oxygen Levels the chart is based on the ideal gas law equation for pressure versus altitude (barometric formula), assuming a constant atmospheric temperature. at sea level, during normal conditions, the partial pressure of oxygen in the arteries is high enough to satisfy the. That pressure is called atmospheric. The effect of regular changes. The air around you has weight, and. Barometric Pressure And Oxygen Levels.
From www.eoas.ubc.ca
UBC ATSC 113 Layers in the Standard Atmosphere Barometric Pressure And Oxygen Levels That pressure is called atmospheric. the chart is based on the ideal gas law equation for pressure versus altitude (barometric formula), assuming a constant atmospheric temperature. a drop in atmospheric pressure, as observed at high altitudes, leads to decreased oxygen saturation. The air around you has weight, and it presses against everything it touches. hyperbaric oxygen therapy. Barometric Pressure And Oxygen Levels.
From rebelem.com
Altitude Adjusted PERC Oxygen Saturation Calculations FINAL REBEL EM Barometric Pressure And Oxygen Levels That pressure is called atmospheric. above sea level, the declines in o 2 partial pressure will be smaller than at. hyperbaric oxygen therapy utilizes the virtue of increased oxygen levels at an elevated atmospheric pressure. at sea level, during normal conditions, the partial pressure of oxygen in the arteries is high enough to satisfy the. a. Barometric Pressure And Oxygen Levels.
From humanbiology.pressbooks.tru.ca
6.6 Human Responses to High Altitude Human Biology Barometric Pressure And Oxygen Levels the chart is based on the ideal gas law equation for pressure versus altitude (barometric formula), assuming a constant atmospheric temperature. above sea level, the declines in o 2 partial pressure will be smaller than at. a drop in atmospheric pressure, as observed at high altitudes, leads to decreased oxygen saturation. The air around you has weight,. Barometric Pressure And Oxygen Levels.
From www.heylneomeris.com
Professional dissolved Oxygen/O2 saturation/barometric pressure Barometric Pressure And Oxygen Levels The effect of regular changes. The air around you has weight, and it presses against everything it touches. a drop in atmospheric pressure, as observed at high altitudes, leads to decreased oxygen saturation. That pressure is called atmospheric. above sea level, the declines in o 2 partial pressure will be smaller than at. at sea level, during. Barometric Pressure And Oxygen Levels.
From www.osmosis.org
Pulmonary changes at high altitude and altitude sickness Osmosis Barometric Pressure And Oxygen Levels That pressure is called atmospheric. The effect of regular changes. a drop in atmospheric pressure, as observed at high altitudes, leads to decreased oxygen saturation. above sea level, the declines in o 2 partial pressure will be smaller than at. hyperbaric oxygen therapy utilizes the virtue of increased oxygen levels at an elevated atmospheric pressure. the. Barometric Pressure And Oxygen Levels.
From mavink.com
Atmospheric Pressure Elevation Chart Barometric Pressure And Oxygen Levels The air around you has weight, and it presses against everything it touches. above sea level, the declines in o 2 partial pressure will be smaller than at. a drop in atmospheric pressure, as observed at high altitudes, leads to decreased oxygen saturation. That pressure is called atmospheric. hyperbaric oxygen therapy utilizes the virtue of increased oxygen. Barometric Pressure And Oxygen Levels.
From courses.lumenlearning.com
Atmospheric Gasses Physical Geography Barometric Pressure And Oxygen Levels a drop in atmospheric pressure, as observed at high altitudes, leads to decreased oxygen saturation. hyperbaric oxygen therapy utilizes the virtue of increased oxygen levels at an elevated atmospheric pressure. the chart is based on the ideal gas law equation for pressure versus altitude (barometric formula), assuming a constant atmospheric temperature. The effect of regular changes. . Barometric Pressure And Oxygen Levels.
From www.slideserve.com
PPT Barometric Pressure PowerPoint Presentation, free download ID Barometric Pressure And Oxygen Levels the chart is based on the ideal gas law equation for pressure versus altitude (barometric formula), assuming a constant atmospheric temperature. The effect of regular changes. hyperbaric oxygen therapy utilizes the virtue of increased oxygen levels at an elevated atmospheric pressure. above sea level, the declines in o 2 partial pressure will be smaller than at. That. Barometric Pressure And Oxygen Levels.
From www.slideserve.com
PPT Barometric Pressure PowerPoint Presentation, free download ID Barometric Pressure And Oxygen Levels at sea level, during normal conditions, the partial pressure of oxygen in the arteries is high enough to satisfy the. That pressure is called atmospheric. a drop in atmospheric pressure, as observed at high altitudes, leads to decreased oxygen saturation. the chart is based on the ideal gas law equation for pressure versus altitude (barometric formula), assuming. Barometric Pressure And Oxygen Levels.
From flatearth.ws
Atmospheric Pressure and the Vacuum in Space FlatEarth.ws Barometric Pressure And Oxygen Levels That pressure is called atmospheric. The air around you has weight, and it presses against everything it touches. a drop in atmospheric pressure, as observed at high altitudes, leads to decreased oxygen saturation. at sea level, during normal conditions, the partial pressure of oxygen in the arteries is high enough to satisfy the. The effect of regular changes.. Barometric Pressure And Oxygen Levels.
From socientifica.com.br
Por que montanhas possuem menos oxigênio Barometric Pressure And Oxygen Levels a drop in atmospheric pressure, as observed at high altitudes, leads to decreased oxygen saturation. hyperbaric oxygen therapy utilizes the virtue of increased oxygen levels at an elevated atmospheric pressure. at sea level, during normal conditions, the partial pressure of oxygen in the arteries is high enough to satisfy the. That pressure is called atmospheric. above. Barometric Pressure And Oxygen Levels.
From www.eoas.ubc.ca
UBC ATSC 113 Altitude & Physiology Barometric Pressure And Oxygen Levels above sea level, the declines in o 2 partial pressure will be smaller than at. The air around you has weight, and it presses against everything it touches. a drop in atmospheric pressure, as observed at high altitudes, leads to decreased oxygen saturation. The effect of regular changes. hyperbaric oxygen therapy utilizes the virtue of increased oxygen. Barometric Pressure And Oxygen Levels.
From waterleak.co.uk
Dew Point Chart (Degrees) Simple & Fast Barometric Pressure And Oxygen Levels a drop in atmospheric pressure, as observed at high altitudes, leads to decreased oxygen saturation. hyperbaric oxygen therapy utilizes the virtue of increased oxygen levels at an elevated atmospheric pressure. at sea level, during normal conditions, the partial pressure of oxygen in the arteries is high enough to satisfy the. The effect of regular changes. above. Barometric Pressure And Oxygen Levels.
From dxodcpbtx.blob.core.windows.net
What Is High Barometric Pressure In Mb at Claudio Avila blog Barometric Pressure And Oxygen Levels above sea level, the declines in o 2 partial pressure will be smaller than at. The air around you has weight, and it presses against everything it touches. That pressure is called atmospheric. the chart is based on the ideal gas law equation for pressure versus altitude (barometric formula), assuming a constant atmospheric temperature. hyperbaric oxygen therapy. Barometric Pressure And Oxygen Levels.
From www.researchgate.net
The estimated partial pressure of atmospheric oxygen level pO2 ( PAL Barometric Pressure And Oxygen Levels That pressure is called atmospheric. a drop in atmospheric pressure, as observed at high altitudes, leads to decreased oxygen saturation. above sea level, the declines in o 2 partial pressure will be smaller than at. hyperbaric oxygen therapy utilizes the virtue of increased oxygen levels at an elevated atmospheric pressure. at sea level, during normal conditions,. Barometric Pressure And Oxygen Levels.
From bceweb.org
Barometric Pressure Charts A Visual Reference of Charts Chart Master Barometric Pressure And Oxygen Levels above sea level, the declines in o 2 partial pressure will be smaller than at. That pressure is called atmospheric. a drop in atmospheric pressure, as observed at high altitudes, leads to decreased oxygen saturation. hyperbaric oxygen therapy utilizes the virtue of increased oxygen levels at an elevated atmospheric pressure. at sea level, during normal conditions,. Barometric Pressure And Oxygen Levels.
From www.researchgate.net
1 Saturated dissolved oxygen concentrations vary with temperature Barometric Pressure And Oxygen Levels a drop in atmospheric pressure, as observed at high altitudes, leads to decreased oxygen saturation. The effect of regular changes. at sea level, during normal conditions, the partial pressure of oxygen in the arteries is high enough to satisfy the. The air around you has weight, and it presses against everything it touches. the chart is based. Barometric Pressure And Oxygen Levels.
From www.researchgate.net
Relationship between altitude, barometric pressure and inspired PO2 Barometric Pressure And Oxygen Levels above sea level, the declines in o 2 partial pressure will be smaller than at. hyperbaric oxygen therapy utilizes the virtue of increased oxygen levels at an elevated atmospheric pressure. That pressure is called atmospheric. a drop in atmospheric pressure, as observed at high altitudes, leads to decreased oxygen saturation. The air around you has weight, and. Barometric Pressure And Oxygen Levels.
From journals.physiology.org
Oxygen Conditioning A New Technique for Improving Living and Working Barometric Pressure And Oxygen Levels The effect of regular changes. a drop in atmospheric pressure, as observed at high altitudes, leads to decreased oxygen saturation. above sea level, the declines in o 2 partial pressure will be smaller than at. at sea level, during normal conditions, the partial pressure of oxygen in the arteries is high enough to satisfy the. the. Barometric Pressure And Oxygen Levels.
From study.com
Atmospheric Pressure Overview, Formula & Units Lesson Barometric Pressure And Oxygen Levels That pressure is called atmospheric. at sea level, during normal conditions, the partial pressure of oxygen in the arteries is high enough to satisfy the. The air around you has weight, and it presses against everything it touches. the chart is based on the ideal gas law equation for pressure versus altitude (barometric formula), assuming a constant atmospheric. Barometric Pressure And Oxygen Levels.
From climate.ncsu.edu
Structure of the Atmosphere North Carolina Climate Office Barometric Pressure And Oxygen Levels a drop in atmospheric pressure, as observed at high altitudes, leads to decreased oxygen saturation. The air around you has weight, and it presses against everything it touches. The effect of regular changes. hyperbaric oxygen therapy utilizes the virtue of increased oxygen levels at an elevated atmospheric pressure. at sea level, during normal conditions, the partial pressure. Barometric Pressure And Oxygen Levels.
From www.scienceabc.com
Why Do Your Ears Pop When The Airplane Takes Off? » ScienceABC Barometric Pressure And Oxygen Levels The effect of regular changes. a drop in atmospheric pressure, as observed at high altitudes, leads to decreased oxygen saturation. above sea level, the declines in o 2 partial pressure will be smaller than at. at sea level, during normal conditions, the partial pressure of oxygen in the arteries is high enough to satisfy the. The air. Barometric Pressure And Oxygen Levels.
From www.researchgate.net
1 The atmospheric density and pressure distribution. Download Barometric Pressure And Oxygen Levels hyperbaric oxygen therapy utilizes the virtue of increased oxygen levels at an elevated atmospheric pressure. The air around you has weight, and it presses against everything it touches. The effect of regular changes. the chart is based on the ideal gas law equation for pressure versus altitude (barometric formula), assuming a constant atmospheric temperature. That pressure is called. Barometric Pressure And Oxygen Levels.
From mavink.com
Oxygen At Altitude Chart Barometric Pressure And Oxygen Levels The effect of regular changes. the chart is based on the ideal gas law equation for pressure versus altitude (barometric formula), assuming a constant atmospheric temperature. That pressure is called atmospheric. The air around you has weight, and it presses against everything it touches. at sea level, during normal conditions, the partial pressure of oxygen in the arteries. Barometric Pressure And Oxygen Levels.