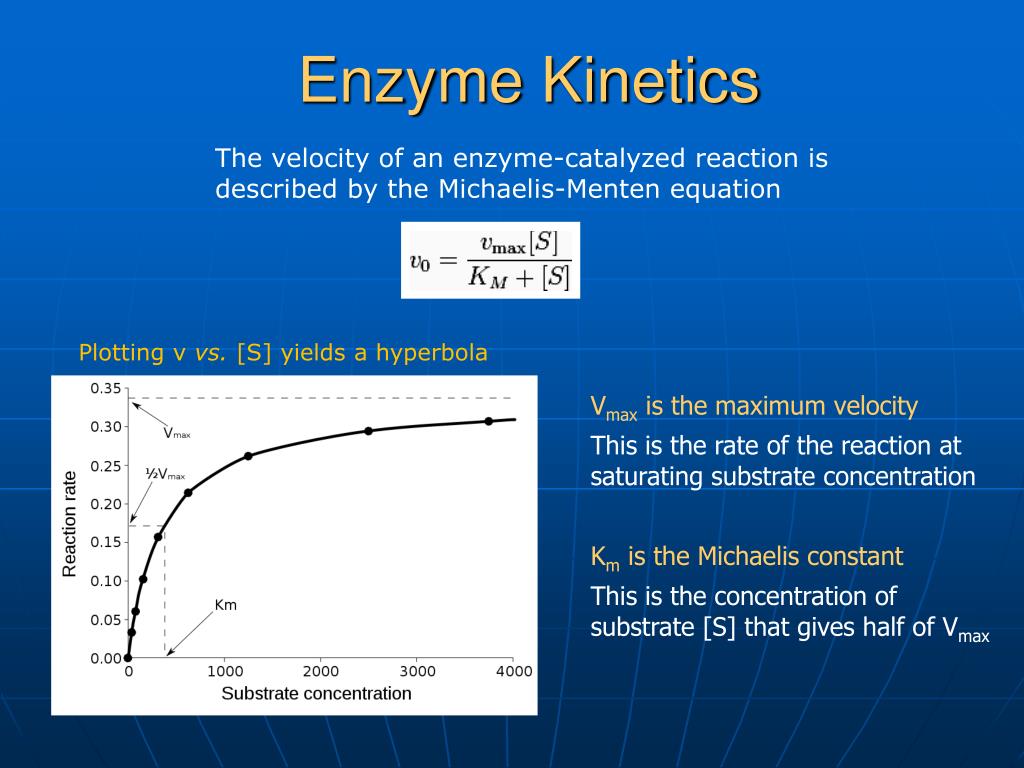
Enzyme Kinetics And Km Value . 10 rows enzyme kinetics is the study of enzyme reaction rates and the conditions which affect them. The value of \(k_m\) is dependent on both the enzyme and the substrate, as well as conditions such as temperature and ph. That implies that the reaction is essentially done as soon as the enzyme and. The effect on kinetics is as if the enzyme were less active (v max is reduced), but that the affinity for substrate is unaffected (k m remains the same) since the substrate binding site is not. The michaelis constant \(k_m\) is the substrate concentration at. \(k_m\) implies that half of the active sites on the enzymes are filled. Different enzymes have different \(k_m\) values. In this article, we will. Some enzymes have k cat /km values around 10 8, indicating that they are diffusion controlled. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by substrate.
from www.slideserve.com
The effect on kinetics is as if the enzyme were less active (v max is reduced), but that the affinity for substrate is unaffected (k m remains the same) since the substrate binding site is not. That implies that the reaction is essentially done as soon as the enzyme and. 10 rows enzyme kinetics is the study of enzyme reaction rates and the conditions which affect them. Some enzymes have k cat /km values around 10 8, indicating that they are diffusion controlled. In this article, we will. Different enzymes have different \(k_m\) values. \(k_m\) implies that half of the active sites on the enzymes are filled. The value of \(k_m\) is dependent on both the enzyme and the substrate, as well as conditions such as temperature and ph. The michaelis constant \(k_m\) is the substrate concentration at. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by substrate.
PPT Enzyme PowerPoint Presentation, free download ID196477
Enzyme Kinetics And Km Value \(k_m\) implies that half of the active sites on the enzymes are filled. In this article, we will. \(k_m\) implies that half of the active sites on the enzymes are filled. That implies that the reaction is essentially done as soon as the enzyme and. The effect on kinetics is as if the enzyme were less active (v max is reduced), but that the affinity for substrate is unaffected (k m remains the same) since the substrate binding site is not. The value of \(k_m\) is dependent on both the enzyme and the substrate, as well as conditions such as temperature and ph. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by substrate. Some enzymes have k cat /km values around 10 8, indicating that they are diffusion controlled. 10 rows enzyme kinetics is the study of enzyme reaction rates and the conditions which affect them. Different enzymes have different \(k_m\) values. The michaelis constant \(k_m\) is the substrate concentration at.
From www.slideserve.com
PPT 513341 Biochemistry I Chapter 7 ENZYME PowerPoint Enzyme Kinetics And Km Value An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by substrate. 10 rows enzyme kinetics is the study of enzyme reaction rates and the conditions which affect them. The michaelis constant \(k_m\) is the substrate concentration at. Some enzymes have k cat /km values around 10 8, indicating that they are diffusion. Enzyme Kinetics And Km Value.
From www.slideserve.com
PPT ENZYME PowerPoint Presentation, free download ID250062 Enzyme Kinetics And Km Value \(k_m\) implies that half of the active sites on the enzymes are filled. That implies that the reaction is essentially done as soon as the enzyme and. Some enzymes have k cat /km values around 10 8, indicating that they are diffusion controlled. 10 rows enzyme kinetics is the study of enzyme reaction rates and the conditions which affect them.. Enzyme Kinetics And Km Value.
From enzymkinetics.com
Enzyme BestCurvFit Software (EZFit, Perrella), Enzyme Kinetics And Km Value \(k_m\) implies that half of the active sites on the enzymes are filled. Different enzymes have different \(k_m\) values. Some enzymes have k cat /km values around 10 8, indicating that they are diffusion controlled. In this article, we will. 10 rows enzyme kinetics is the study of enzyme reaction rates and the conditions which affect them. The value of. Enzyme Kinetics And Km Value.
From www.youtube.com
Constants Km & Vmax YouTube Enzyme Kinetics And Km Value The value of \(k_m\) is dependent on both the enzyme and the substrate, as well as conditions such as temperature and ph. Different enzymes have different \(k_m\) values. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by substrate. The michaelis constant \(k_m\) is the substrate concentration at. In this article, we. Enzyme Kinetics And Km Value.
From www.sqadia.com
Enzyme I Introduction Enzyme Kinetics And Km Value 10 rows enzyme kinetics is the study of enzyme reaction rates and the conditions which affect them. Different enzymes have different \(k_m\) values. The effect on kinetics is as if the enzyme were less active (v max is reduced), but that the affinity for substrate is unaffected (k m remains the same) since the substrate binding site is not. That. Enzyme Kinetics And Km Value.
From www.slideserve.com
PPT Enzyme I PowerPoint Presentation, free download ID298555 Enzyme Kinetics And Km Value The michaelis constant \(k_m\) is the substrate concentration at. The value of \(k_m\) is dependent on both the enzyme and the substrate, as well as conditions such as temperature and ph. 10 rows enzyme kinetics is the study of enzyme reaction rates and the conditions which affect them. The effect on kinetics is as if the enzyme were less active. Enzyme Kinetics And Km Value.
From jackwestin.com
Control Of Enzyme Activity MCAT Content Enzyme Kinetics And Km Value \(k_m\) implies that half of the active sites on the enzymes are filled. 10 rows enzyme kinetics is the study of enzyme reaction rates and the conditions which affect them. The michaelis constant \(k_m\) is the substrate concentration at. The effect on kinetics is as if the enzyme were less active (v max is reduced), but that the affinity for. Enzyme Kinetics And Km Value.
From www.slideserve.com
PPT 513341 Biochemistry I Chapter 7 ENZYME PowerPoint Enzyme Kinetics And Km Value The michaelis constant \(k_m\) is the substrate concentration at. Different enzymes have different \(k_m\) values. 10 rows enzyme kinetics is the study of enzyme reaction rates and the conditions which affect them. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by substrate. In this article, we will. The value of \(k_m\). Enzyme Kinetics And Km Value.
From www.numerade.com
SOLVED Enzyme Quick recap What happens at zero [S]? E + 5 ES Enzyme Kinetics And Km Value Different enzymes have different \(k_m\) values. The value of \(k_m\) is dependent on both the enzyme and the substrate, as well as conditions such as temperature and ph. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by substrate. Some enzymes have k cat /km values around 10 8, indicating that they. Enzyme Kinetics And Km Value.
From easybiologyclass.com
What is Km in Enzyme EasyBiologyClass Enzyme Kinetics And Km Value \(k_m\) implies that half of the active sites on the enzymes are filled. 10 rows enzyme kinetics is the study of enzyme reaction rates and the conditions which affect them. Different enzymes have different \(k_m\) values. That implies that the reaction is essentially done as soon as the enzyme and. The michaelis constant \(k_m\) is the substrate concentration at. An. Enzyme Kinetics And Km Value.
From www.chegg.com
Solved Experiment 7 Enzyme Determination of Km Enzyme Kinetics And Km Value Different enzymes have different \(k_m\) values. The effect on kinetics is as if the enzyme were less active (v max is reduced), but that the affinity for substrate is unaffected (k m remains the same) since the substrate binding site is not. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by. Enzyme Kinetics And Km Value.
From www.slideserve.com
PPT Chapter 13 Enzyme PowerPoint Presentation, free download Enzyme Kinetics And Km Value 10 rows enzyme kinetics is the study of enzyme reaction rates and the conditions which affect them. \(k_m\) implies that half of the active sites on the enzymes are filled. In this article, we will. That implies that the reaction is essentially done as soon as the enzyme and. An enzyme's k m describes the substrate concentration at which half. Enzyme Kinetics And Km Value.
From www.slideserve.com
PPT Biochemical Reaction Rate Enzyme PowerPoint Enzyme Kinetics And Km Value The effect on kinetics is as if the enzyme were less active (v max is reduced), but that the affinity for substrate is unaffected (k m remains the same) since the substrate binding site is not. Different enzymes have different \(k_m\) values. The michaelis constant \(k_m\) is the substrate concentration at. Some enzymes have k cat /km values around 10. Enzyme Kinetics And Km Value.
From teachmephysiology.com
Enzyme Structure Function MichaelisMenten Enzyme Kinetics And Km Value The effect on kinetics is as if the enzyme were less active (v max is reduced), but that the affinity for substrate is unaffected (k m remains the same) since the substrate binding site is not. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by substrate. In this article, we will.. Enzyme Kinetics And Km Value.
From wingkiwong.net
Areas of Interest Page 2 Wing Ki (Catherine) Wong Enzyme Kinetics And Km Value Some enzymes have k cat /km values around 10 8, indicating that they are diffusion controlled. The value of \(k_m\) is dependent on both the enzyme and the substrate, as well as conditions such as temperature and ph. In this article, we will. Different enzymes have different \(k_m\) values. The michaelis constant \(k_m\) is the substrate concentration at. That implies. Enzyme Kinetics And Km Value.
From www.youtube.com
AS Biology The MichaelisMenten Constant (Km) YouTube Enzyme Kinetics And Km Value In this article, we will. The effect on kinetics is as if the enzyme were less active (v max is reduced), but that the affinity for substrate is unaffected (k m remains the same) since the substrate binding site is not. 10 rows enzyme kinetics is the study of enzyme reaction rates and the conditions which affect them. That implies. Enzyme Kinetics And Km Value.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID1477171 Enzyme Kinetics And Km Value In this article, we will. The value of \(k_m\) is dependent on both the enzyme and the substrate, as well as conditions such as temperature and ph. Different enzymes have different \(k_m\) values. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by substrate. \(k_m\) implies that half of the active sites. Enzyme Kinetics And Km Value.
From www.slideserve.com
PPT Chapter 6.3 Enzyme PowerPoint Presentation, free Enzyme Kinetics And Km Value That implies that the reaction is essentially done as soon as the enzyme and. \(k_m\) implies that half of the active sites on the enzymes are filled. Different enzymes have different \(k_m\) values. The effect on kinetics is as if the enzyme were less active (v max is reduced), but that the affinity for substrate is unaffected (k m remains. Enzyme Kinetics And Km Value.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID9379885 Enzyme Kinetics And Km Value Some enzymes have k cat /km values around 10 8, indicating that they are diffusion controlled. Different enzymes have different \(k_m\) values. 10 rows enzyme kinetics is the study of enzyme reaction rates and the conditions which affect them. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by substrate. The effect. Enzyme Kinetics And Km Value.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID6630235 Enzyme Kinetics And Km Value The value of \(k_m\) is dependent on both the enzyme and the substrate, as well as conditions such as temperature and ph. That implies that the reaction is essentially done as soon as the enzyme and. The michaelis constant \(k_m\) is the substrate concentration at. In this article, we will. 10 rows enzyme kinetics is the study of enzyme reaction. Enzyme Kinetics And Km Value.
From www.youtube.com
B.7 Vmax and Km (HL) YouTube Enzyme Kinetics And Km Value In this article, we will. \(k_m\) implies that half of the active sites on the enzymes are filled. The value of \(k_m\) is dependent on both the enzyme and the substrate, as well as conditions such as temperature and ph. The michaelis constant \(k_m\) is the substrate concentration at. That implies that the reaction is essentially done as soon as. Enzyme Kinetics And Km Value.
From www.slideserve.com
PPT Enzymes, con't. PowerPoint Presentation, free download ID1477385 Enzyme Kinetics And Km Value 10 rows enzyme kinetics is the study of enzyme reaction rates and the conditions which affect them. That implies that the reaction is essentially done as soon as the enzyme and. The value of \(k_m\) is dependent on both the enzyme and the substrate, as well as conditions such as temperature and ph. Some enzymes have k cat /km values. Enzyme Kinetics And Km Value.
From www.slideserve.com
PPT Chapter 6.3 Enzyme PowerPoint Presentation, free Enzyme Kinetics And Km Value Some enzymes have k cat /km values around 10 8, indicating that they are diffusion controlled. The value of \(k_m\) is dependent on both the enzyme and the substrate, as well as conditions such as temperature and ph. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by substrate. That implies that. Enzyme Kinetics And Km Value.
From www.slideserve.com
PPT 513341 Biochemistry I Chapter 7 ENZYME PowerPoint Enzyme Kinetics And Km Value 10 rows enzyme kinetics is the study of enzyme reaction rates and the conditions which affect them. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by substrate. The michaelis constant \(k_m\) is the substrate concentration at. Some enzymes have k cat /km values around 10 8, indicating that they are diffusion. Enzyme Kinetics And Km Value.
From www.slideserve.com
PPT Chapter 6 Enzyme PowerPoint Presentation, free download ID5692414 Enzyme Kinetics And Km Value 10 rows enzyme kinetics is the study of enzyme reaction rates and the conditions which affect them. The michaelis constant \(k_m\) is the substrate concentration at. Different enzymes have different \(k_m\) values. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by substrate. The effect on kinetics is as if the enzyme. Enzyme Kinetics And Km Value.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID903733 Enzyme Kinetics And Km Value The value of \(k_m\) is dependent on both the enzyme and the substrate, as well as conditions such as temperature and ph. The effect on kinetics is as if the enzyme were less active (v max is reduced), but that the affinity for substrate is unaffected (k m remains the same) since the substrate binding site is not. 10 rows. Enzyme Kinetics And Km Value.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID1477171 Enzyme Kinetics And Km Value 10 rows enzyme kinetics is the study of enzyme reaction rates and the conditions which affect them. The value of \(k_m\) is dependent on both the enzyme and the substrate, as well as conditions such as temperature and ph. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by substrate. The michaelis. Enzyme Kinetics And Km Value.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID903733 Enzyme Kinetics And Km Value \(k_m\) implies that half of the active sites on the enzymes are filled. The effect on kinetics is as if the enzyme were less active (v max is reduced), but that the affinity for substrate is unaffected (k m remains the same) since the substrate binding site is not. The michaelis constant \(k_m\) is the substrate concentration at. Different enzymes. Enzyme Kinetics And Km Value.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID2045841 Enzyme Kinetics And Km Value Some enzymes have k cat /km values around 10 8, indicating that they are diffusion controlled. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by substrate. The michaelis constant \(k_m\) is the substrate concentration at. In this article, we will. The value of \(k_m\) is dependent on both the enzyme and. Enzyme Kinetics And Km Value.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID903733 Enzyme Kinetics And Km Value That implies that the reaction is essentially done as soon as the enzyme and. 10 rows enzyme kinetics is the study of enzyme reaction rates and the conditions which affect them. Some enzymes have k cat /km values around 10 8, indicating that they are diffusion controlled. \(k_m\) implies that half of the active sites on the enzymes are filled.. Enzyme Kinetics And Km Value.
From plantphys.info
Enzyme Enzyme Kinetics And Km Value Some enzymes have k cat /km values around 10 8, indicating that they are diffusion controlled. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by substrate. In this article, we will. 10 rows enzyme kinetics is the study of enzyme reaction rates and the conditions which affect them. The michaelis constant. Enzyme Kinetics And Km Value.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID196477 Enzyme Kinetics And Km Value That implies that the reaction is essentially done as soon as the enzyme and. \(k_m\) implies that half of the active sites on the enzymes are filled. The michaelis constant \(k_m\) is the substrate concentration at. The effect on kinetics is as if the enzyme were less active (v max is reduced), but that the affinity for substrate is unaffected. Enzyme Kinetics And Km Value.
From www.biologyonline.com
Vmax Definition and Examples Biology Online Dictionary Enzyme Kinetics And Km Value The value of \(k_m\) is dependent on both the enzyme and the substrate, as well as conditions such as temperature and ph. Different enzymes have different \(k_m\) values. \(k_m\) implies that half of the active sites on the enzymes are filled. That implies that the reaction is essentially done as soon as the enzyme and. 10 rows enzyme kinetics is. Enzyme Kinetics And Km Value.
From web.hktechnical.com
Enzyme and km value B.Pharmacy 2nd Semester Lecture Notes PDF Enzyme Kinetics And Km Value \(k_m\) implies that half of the active sites on the enzymes are filled. Some enzymes have k cat /km values around 10 8, indicating that they are diffusion controlled. Different enzymes have different \(k_m\) values. That implies that the reaction is essentially done as soon as the enzyme and. The effect on kinetics is as if the enzyme were less. Enzyme Kinetics And Km Value.
From courses.lumenlearning.com
Enzymes OpenStax Biology 2e Enzyme Kinetics And Km Value An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by substrate. In this article, we will. That implies that the reaction is essentially done as soon as the enzyme and. \(k_m\) implies that half of the active sites on the enzymes are filled. The effect on kinetics is as if the enzyme. Enzyme Kinetics And Km Value.