Essential Requirements Checklist . The general safety and performance requirements check list shall be a controlled document. This document provides harmonized essential principles that should be fulfilled in the design and manufacturing of medical devices and ivd. A checklist of essential requirements for medical devices based on eu regulations and directives. Devices must be designed and manufactured in such a way as to reduce, as much as possible, risks posed by the unintentional ingress of. The checklist covers topics such as general. Generally a gspr checklist is considered the best way to document these requirements. Applicability of the general safety. Notified bodies have tried to fill this gap by insisting on an essential requirements checklist. Many manufacturers have ‘essential requirements checklists’ embedded in their new product development processes. As compliance with the ‘essential requirements (ers)’ is the keystone for establishing conformity with the medical device directive (mdd,. This checklist has now been.
from flevy.com
Applicability of the general safety. The general safety and performance requirements check list shall be a controlled document. Generally a gspr checklist is considered the best way to document these requirements. The checklist covers topics such as general. As compliance with the ‘essential requirements (ers)’ is the keystone for establishing conformity with the medical device directive (mdd,. A checklist of essential requirements for medical devices based on eu regulations and directives. Notified bodies have tried to fill this gap by insisting on an essential requirements checklist. Many manufacturers have ‘essential requirements checklists’ embedded in their new product development processes. This checklist has now been. Devices must be designed and manufactured in such a way as to reduce, as much as possible, risks posed by the unintentional ingress of.
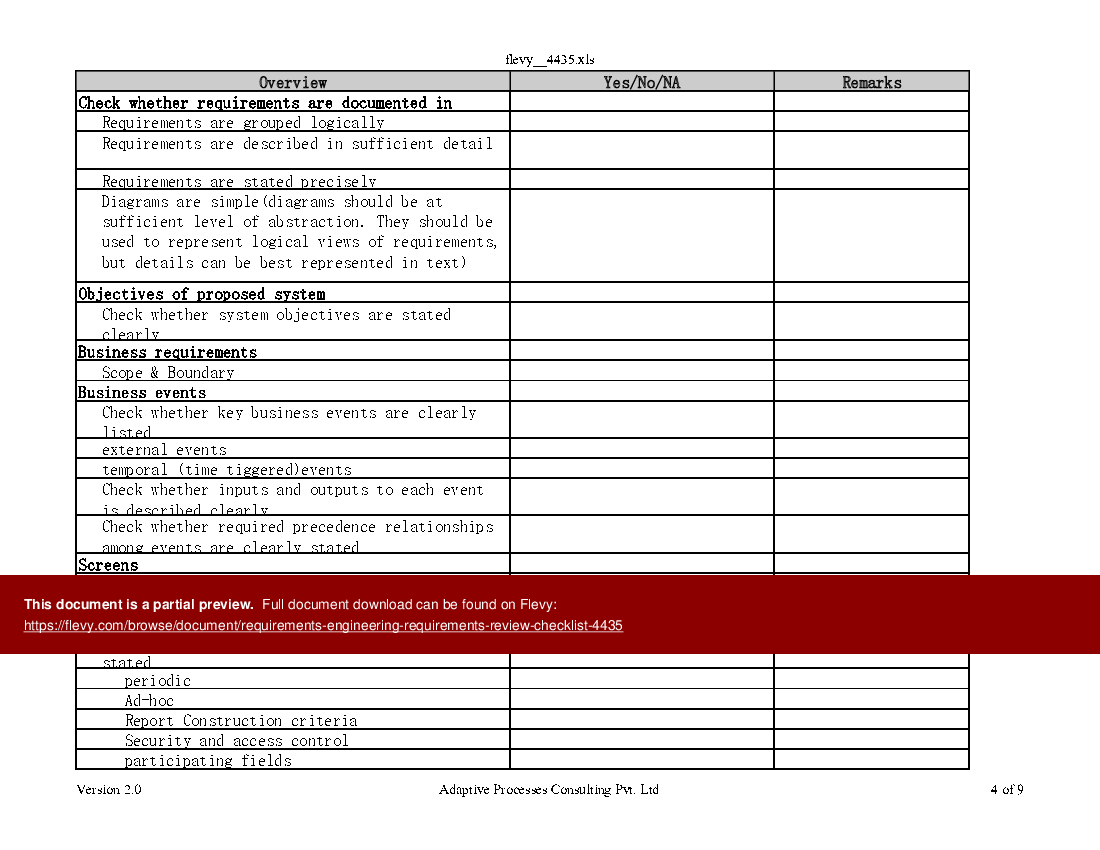
Excel Template Requirements engineering Requirements Review Checklist
Essential Requirements Checklist This checklist has now been. The checklist covers topics such as general. Many manufacturers have ‘essential requirements checklists’ embedded in their new product development processes. Notified bodies have tried to fill this gap by insisting on an essential requirements checklist. A checklist of essential requirements for medical devices based on eu regulations and directives. Generally a gspr checklist is considered the best way to document these requirements. Applicability of the general safety. This document provides harmonized essential principles that should be fulfilled in the design and manufacturing of medical devices and ivd. Devices must be designed and manufactured in such a way as to reduce, as much as possible, risks posed by the unintentional ingress of. As compliance with the ‘essential requirements (ers)’ is the keystone for establishing conformity with the medical device directive (mdd,. This checklist has now been. The general safety and performance requirements check list shall be a controlled document.
From www.jamasoftware.com
Checklist Selecting a Requirements Management Tool Jama Software Essential Requirements Checklist Many manufacturers have ‘essential requirements checklists’ embedded in their new product development processes. Generally a gspr checklist is considered the best way to document these requirements. A checklist of essential requirements for medical devices based on eu regulations and directives. The general safety and performance requirements check list shall be a controlled document. This checklist has now been. Applicability of. Essential Requirements Checklist.
From dokumen.tips
(DOCX) · viewEssential Requirements Checklist according to COUNCIL Essential Requirements Checklist This document provides harmonized essential principles that should be fulfilled in the design and manufacturing of medical devices and ivd. As compliance with the ‘essential requirements (ers)’ is the keystone for establishing conformity with the medical device directive (mdd,. A checklist of essential requirements for medical devices based on eu regulations and directives. Devices must be designed and manufactured in. Essential Requirements Checklist.
From www.examples.com
Project Requirement Checklist 10+ Examples, Format, Pdf, Tips Essential Requirements Checklist This document provides harmonized essential principles that should be fulfilled in the design and manufacturing of medical devices and ivd. The checklist covers topics such as general. Many manufacturers have ‘essential requirements checklists’ embedded in their new product development processes. Generally a gspr checklist is considered the best way to document these requirements. Applicability of the general safety. A checklist. Essential Requirements Checklist.
From easymedicaldevice.com
Best Tips ISO 13485 procedures with our free template (Version 2016) Essential Requirements Checklist The general safety and performance requirements check list shall be a controlled document. The checklist covers topics such as general. This document provides harmonized essential principles that should be fulfilled in the design and manufacturing of medical devices and ivd. This checklist has now been. Applicability of the general safety. As compliance with the ‘essential requirements (ers)’ is the keystone. Essential Requirements Checklist.
From www.scribd.com
Generic Essential Requirements Checklist Risk Management Essential Requirements Checklist This document provides harmonized essential principles that should be fulfilled in the design and manufacturing of medical devices and ivd. Applicability of the general safety. Generally a gspr checklist is considered the best way to document these requirements. As compliance with the ‘essential requirements (ers)’ is the keystone for establishing conformity with the medical device directive (mdd,. The checklist covers. Essential Requirements Checklist.
From www.rimsys.io
Australian Essential Principles Essential Requirements Checklist A checklist of essential requirements for medical devices based on eu regulations and directives. Notified bodies have tried to fill this gap by insisting on an essential requirements checklist. Many manufacturers have ‘essential requirements checklists’ embedded in their new product development processes. This checklist has now been. Devices must be designed and manufactured in such a way as to reduce,. Essential Requirements Checklist.
From www.johner-institute.com
The MDR's Usability Requirements Essential Requirements Checklist The general safety and performance requirements check list shall be a controlled document. This document provides harmonized essential principles that should be fulfilled in the design and manufacturing of medical devices and ivd. Generally a gspr checklist is considered the best way to document these requirements. The checklist covers topics such as general. Devices must be designed and manufactured in. Essential Requirements Checklist.
From www.slideserve.com
PPT The Impact of Regulations on Medical Device Design PowerPoint Essential Requirements Checklist Devices must be designed and manufactured in such a way as to reduce, as much as possible, risks posed by the unintentional ingress of. The general safety and performance requirements check list shall be a controlled document. Notified bodies have tried to fill this gap by insisting on an essential requirements checklist. Generally a gspr checklist is considered the best. Essential Requirements Checklist.
From www.scribd.com
IVD Checklist PDF Medical Device Specification (Technical Standard) Essential Requirements Checklist This document provides harmonized essential principles that should be fulfilled in the design and manufacturing of medical devices and ivd. Notified bodies have tried to fill this gap by insisting on an essential requirements checklist. This checklist has now been. Generally a gspr checklist is considered the best way to document these requirements. Devices must be designed and manufactured in. Essential Requirements Checklist.
From www.scribd.com
European MDR Readiness Checklist Fillable PDF Quality Management Essential Requirements Checklist Notified bodies have tried to fill this gap by insisting on an essential requirements checklist. Applicability of the general safety. The checklist covers topics such as general. Many manufacturers have ‘essential requirements checklists’ embedded in their new product development processes. The general safety and performance requirements check list shall be a controlled document. As compliance with the ‘essential requirements (ers)’. Essential Requirements Checklist.
From www.examples.com
Business Requirements Document 39+ Examples, Format, Pdf Essential Requirements Checklist Devices must be designed and manufactured in such a way as to reduce, as much as possible, risks posed by the unintentional ingress of. The general safety and performance requirements check list shall be a controlled document. This document provides harmonized essential principles that should be fulfilled in the design and manufacturing of medical devices and ivd. As compliance with. Essential Requirements Checklist.
From ordicu.com
Project Management Software Requirements Checklist Template (2023) Essential Requirements Checklist Generally a gspr checklist is considered the best way to document these requirements. This checklist has now been. Devices must be designed and manufactured in such a way as to reduce, as much as possible, risks posed by the unintentional ingress of. The general safety and performance requirements check list shall be a controlled document. Applicability of the general safety.. Essential Requirements Checklist.
From www.researchgate.net
Essential requirement checklist. Download Scientific Diagram Essential Requirements Checklist Generally a gspr checklist is considered the best way to document these requirements. Many manufacturers have ‘essential requirements checklists’ embedded in their new product development processes. As compliance with the ‘essential requirements (ers)’ is the keystone for establishing conformity with the medical device directive (mdd,. A checklist of essential requirements for medical devices based on eu regulations and directives. Notified. Essential Requirements Checklist.
From www.examples.com
Project Requirement Checklist 10+ Examples, Format, Pdf, Tips Essential Requirements Checklist The checklist covers topics such as general. Notified bodies have tried to fill this gap by insisting on an essential requirements checklist. Applicability of the general safety. The general safety and performance requirements check list shall be a controlled document. A checklist of essential requirements for medical devices based on eu regulations and directives. Generally a gspr checklist is considered. Essential Requirements Checklist.
From checklist.gg
Saas product requirements checklist checklist.gg Essential Requirements Checklist Many manufacturers have ‘essential requirements checklists’ embedded in their new product development processes. Devices must be designed and manufactured in such a way as to reduce, as much as possible, risks posed by the unintentional ingress of. The general safety and performance requirements check list shall be a controlled document. This checklist has now been. Notified bodies have tried to. Essential Requirements Checklist.
From studylib.net
GN16 Essential Principles Checklist Template Essential Requirements Checklist Notified bodies have tried to fill this gap by insisting on an essential requirements checklist. This checklist has now been. The general safety and performance requirements check list shall be a controlled document. Applicability of the general safety. Devices must be designed and manufactured in such a way as to reduce, as much as possible, risks posed by the unintentional. Essential Requirements Checklist.
From qmsregs.com
Essential Requirements Checklist Template UKCA Marking Essential Requirements Checklist Notified bodies have tried to fill this gap by insisting on an essential requirements checklist. The checklist covers topics such as general. Applicability of the general safety. This checklist has now been. This document provides harmonized essential principles that should be fulfilled in the design and manufacturing of medical devices and ivd. The general safety and performance requirements check list. Essential Requirements Checklist.
From www.examples.com
Project Requirement Checklist 10+ Examples, Format, Pdf, Tips Essential Requirements Checklist Generally a gspr checklist is considered the best way to document these requirements. This checklist has now been. A checklist of essential requirements for medical devices based on eu regulations and directives. Applicability of the general safety. This document provides harmonized essential principles that should be fulfilled in the design and manufacturing of medical devices and ivd. The checklist covers. Essential Requirements Checklist.
From gbu-taganskij.ru
9342EC ER Checklist Example PDF Medical Device Risk, 44 OFF Essential Requirements Checklist This document provides harmonized essential principles that should be fulfilled in the design and manufacturing of medical devices and ivd. Many manufacturers have ‘essential requirements checklists’ embedded in their new product development processes. A checklist of essential requirements for medical devices based on eu regulations and directives. This checklist has now been. Generally a gspr checklist is considered the best. Essential Requirements Checklist.
From scientistssanctuary.com
ESSENTIAL REQUIREMENTS TEMPLATE Scientists Sanctuary Essential Requirements Checklist Devices must be designed and manufactured in such a way as to reduce, as much as possible, risks posed by the unintentional ingress of. Generally a gspr checklist is considered the best way to document these requirements. Applicability of the general safety. The general safety and performance requirements check list shall be a controlled document. This checklist has now been.. Essential Requirements Checklist.
From www.wendangwang.com
Essential Requirements Checklist of the MDD(07 47 EC)基本要求检查表(中英文)_word Essential Requirements Checklist The checklist covers topics such as general. This checklist has now been. As compliance with the ‘essential requirements (ers)’ is the keystone for establishing conformity with the medical device directive (mdd,. Devices must be designed and manufactured in such a way as to reduce, as much as possible, risks posed by the unintentional ingress of. Notified bodies have tried to. Essential Requirements Checklist.
From www.examples.com
Project Requirement Checklist 10+ Examples, Format, Pdf, Tips Essential Requirements Checklist Applicability of the general safety. This checklist has now been. Devices must be designed and manufactured in such a way as to reduce, as much as possible, risks posed by the unintentional ingress of. Generally a gspr checklist is considered the best way to document these requirements. This document provides harmonized essential principles that should be fulfilled in the design. Essential Requirements Checklist.
From www.scribd.com
DX032851 Essential Requirements Checklist V4 (IVDD Annex I of IVD Essential Requirements Checklist Many manufacturers have ‘essential requirements checklists’ embedded in their new product development processes. Notified bodies have tried to fill this gap by insisting on an essential requirements checklist. The general safety and performance requirements check list shall be a controlled document. Applicability of the general safety. Generally a gspr checklist is considered the best way to document these requirements. As. Essential Requirements Checklist.
From www.examples.com
Project Requirement Checklist 10+ Examples, Format, Pdf, Tips Essential Requirements Checklist This document provides harmonized essential principles that should be fulfilled in the design and manufacturing of medical devices and ivd. A checklist of essential requirements for medical devices based on eu regulations and directives. The general safety and performance requirements check list shall be a controlled document. Many manufacturers have ‘essential requirements checklists’ embedded in their new product development processes.. Essential Requirements Checklist.
From www.examples.com
Project Requirement Checklist 10+ Examples, Format, Pdf, Tips Essential Requirements Checklist Many manufacturers have ‘essential requirements checklists’ embedded in their new product development processes. The general safety and performance requirements check list shall be a controlled document. A checklist of essential requirements for medical devices based on eu regulations and directives. The checklist covers topics such as general. This checklist has now been. Applicability of the general safety. Notified bodies have. Essential Requirements Checklist.
From www.gretaforag.com
Essential Requirements Checklist Template Essential Requirements Checklist This checklist has now been. A checklist of essential requirements for medical devices based on eu regulations and directives. The general safety and performance requirements check list shall be a controlled document. Notified bodies have tried to fill this gap by insisting on an essential requirements checklist. Generally a gspr checklist is considered the best way to document these requirements.. Essential Requirements Checklist.
From wwww.todolistsoft.com
Business Requirements Checklist To Do List, Organizer, Checklist, PIM Essential Requirements Checklist Applicability of the general safety. As compliance with the ‘essential requirements (ers)’ is the keystone for establishing conformity with the medical device directive (mdd,. Devices must be designed and manufactured in such a way as to reduce, as much as possible, risks posed by the unintentional ingress of. Generally a gspr checklist is considered the best way to document these. Essential Requirements Checklist.
From www.slideteam.net
Customer Relationship Management Toolkit Checklist For CRM Business Essential Requirements Checklist This checklist has now been. As compliance with the ‘essential requirements (ers)’ is the keystone for establishing conformity with the medical device directive (mdd,. Notified bodies have tried to fill this gap by insisting on an essential requirements checklist. Many manufacturers have ‘essential requirements checklists’ embedded in their new product development processes. The checklist covers topics such as general. Devices. Essential Requirements Checklist.
From studylib.net
Medical devices essential principles checklist Essential Requirements Checklist This checklist has now been. A checklist of essential requirements for medical devices based on eu regulations and directives. Notified bodies have tried to fill this gap by insisting on an essential requirements checklist. Devices must be designed and manufactured in such a way as to reduce, as much as possible, risks posed by the unintentional ingress of. Generally a. Essential Requirements Checklist.
From www.examples.com
Checklist Examples 79+ in DOC Examples Essential Requirements Checklist Many manufacturers have ‘essential requirements checklists’ embedded in their new product development processes. Applicability of the general safety. This checklist has now been. A checklist of essential requirements for medical devices based on eu regulations and directives. The general safety and performance requirements check list shall be a controlled document. This document provides harmonized essential principles that should be fulfilled. Essential Requirements Checklist.
From printabletemplate.concejomunicipaldechinu.gov.co
Mdr Gspr Checklist Template Essential Requirements Checklist Devices must be designed and manufactured in such a way as to reduce, as much as possible, risks posed by the unintentional ingress of. A checklist of essential requirements for medical devices based on eu regulations and directives. Applicability of the general safety. This document provides harmonized essential principles that should be fulfilled in the design and manufacturing of medical. Essential Requirements Checklist.
From flevy.com
Excel Template Requirements engineering Requirements Review Checklist Essential Requirements Checklist As compliance with the ‘essential requirements (ers)’ is the keystone for establishing conformity with the medical device directive (mdd,. Many manufacturers have ‘essential requirements checklists’ embedded in their new product development processes. This checklist has now been. This document provides harmonized essential principles that should be fulfilled in the design and manufacturing of medical devices and ivd. Applicability of the. Essential Requirements Checklist.
From patientguard.com
IVD Directive Essential Requirements Checklist Essential Requirements Checklist Notified bodies have tried to fill this gap by insisting on an essential requirements checklist. The checklist covers topics such as general. Many manufacturers have ‘essential requirements checklists’ embedded in their new product development processes. As compliance with the ‘essential requirements (ers)’ is the keystone for establishing conformity with the medical device directive (mdd,. Generally a gspr checklist is considered. Essential Requirements Checklist.
From www.mianfeiwendang.com
Essential Requirements Checklist of the MDD(07 47 EC)基本要求检查表(中英文)_word Essential Requirements Checklist Generally a gspr checklist is considered the best way to document these requirements. The general safety and performance requirements check list shall be a controlled document. As compliance with the ‘essential requirements (ers)’ is the keystone for establishing conformity with the medical device directive (mdd,. Devices must be designed and manufactured in such a way as to reduce, as much. Essential Requirements Checklist.
From www.examples.com
Project Requirement Checklist 10+ Examples, Format, Pdf, Tips Essential Requirements Checklist The general safety and performance requirements check list shall be a controlled document. Generally a gspr checklist is considered the best way to document these requirements. This document provides harmonized essential principles that should be fulfilled in the design and manufacturing of medical devices and ivd. A checklist of essential requirements for medical devices based on eu regulations and directives.. Essential Requirements Checklist.