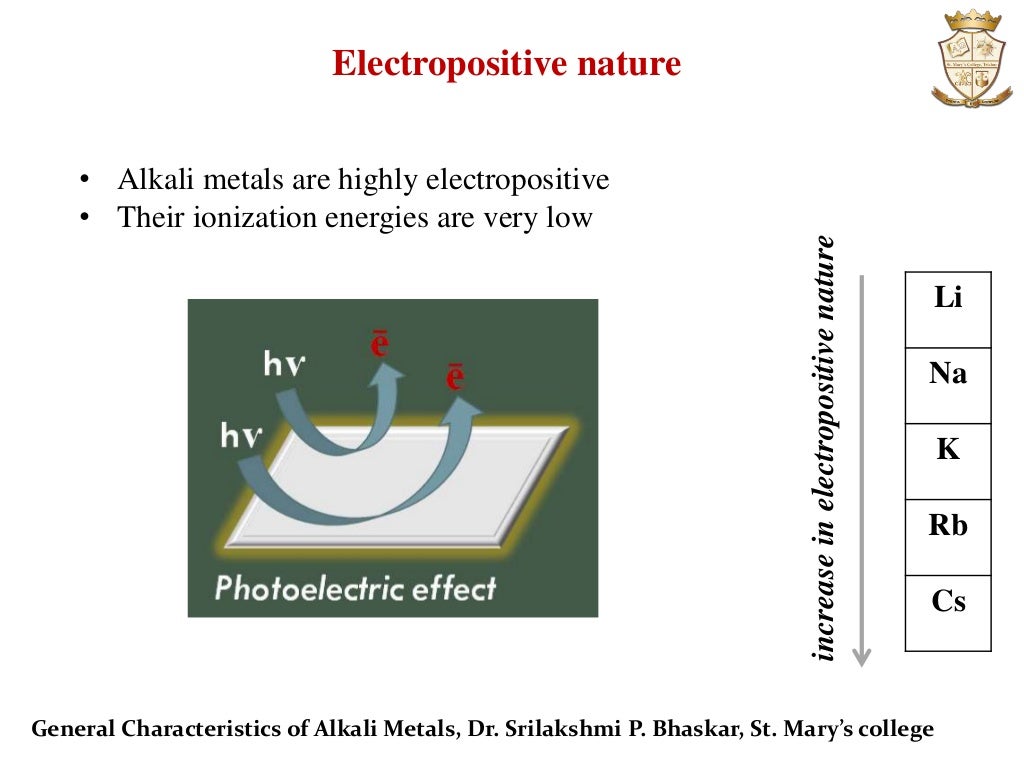
Alkali Characteristics . The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. The alkali metals are a group of elements in the periodic table with similar properties: The alkali metals are potent reductants whose chemistry is largely that of ionic compounds containing the m+ ion. The key characteristic these elements share in common is that they all have one. Alkali metals have only a weak tendency to form complexes with simple lewis bases. The alkali metals are the elements located in group ia of the periodic table (the first column). Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g., thermal and electrical conductivity.
from www.slideshare.net
The alkali metals are the elements located in group ia of the periodic table (the first column). The alkali metals are potent reductants whose chemistry is largely that of ionic compounds containing the m+ ion. The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. The alkali metals are a group of elements in the periodic table with similar properties: Alkali metals have only a weak tendency to form complexes with simple lewis bases. Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g., thermal and electrical conductivity. The key characteristic these elements share in common is that they all have one.
ChemistryGeneral Characteristics Of Alkali Metals
Alkali Characteristics The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. The alkali metals are the elements located in group ia of the periodic table (the first column). The alkali metals are potent reductants whose chemistry is largely that of ionic compounds containing the m+ ion. The alkali metals are a group of elements in the periodic table with similar properties: The key characteristic these elements share in common is that they all have one. Alkali metals have only a weak tendency to form complexes with simple lewis bases. Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g., thermal and electrical conductivity. The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals.
From utedzz.blogspot.com
Periodic Table Alkali Metals Periodic Table Timeline Alkali Characteristics The alkali metals are the elements located in group ia of the periodic table (the first column). Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g., thermal and electrical conductivity. Alkali metals have only a weak tendency to form complexes with simple lewis bases. The key characteristic these elements share in common is. Alkali Characteristics.
From www.sliderbase.com
Group 1&2 Presentation Chemistry Alkali Characteristics The alkali metals are potent reductants whose chemistry is largely that of ionic compounds containing the m+ ion. The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g., thermal and electrical conductivity.. Alkali Characteristics.
From byjus.com
Alkaline Earth Metals Occurrence and Extraction,Physical Properties Alkali Characteristics Alkali metals have only a weak tendency to form complexes with simple lewis bases. Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g., thermal and electrical conductivity. The alkali metals are the elements located in group ia of the periodic table (the first column). The alkali metals are a group of elements in. Alkali Characteristics.
From study.com
Alkali Metals Definition, Properties & Characteristics Lesson Alkali Characteristics The key characteristic these elements share in common is that they all have one. The alkali metals are potent reductants whose chemistry is largely that of ionic compounds containing the m+ ion. The alkali metals are the elements located in group ia of the periodic table (the first column). The alkali metals are a group of elements in the periodic. Alkali Characteristics.
From www.sliderbase.com
Element Classes Presentation Chemistry Alkali Characteristics The alkali metals are potent reductants whose chemistry is largely that of ionic compounds containing the m+ ion. Alkali metals have only a weak tendency to form complexes with simple lewis bases. The key characteristic these elements share in common is that they all have one. The alkali metals are a group of elements in the periodic table with similar. Alkali Characteristics.
From issuu.com
Chemistry Alkali Metals by AshfordSchool Issuu Alkali Characteristics Alkali metals have only a weak tendency to form complexes with simple lewis bases. The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. The alkali metals are potent reductants whose chemistry is largely that of ionic compounds containing the m+ ion. The key characteristic these elements share. Alkali Characteristics.
From pediaa.com
Difference Between Alkali Metals and Alkaline Earth Metals Definition Alkali Characteristics The alkali metals are potent reductants whose chemistry is largely that of ionic compounds containing the m+ ion. The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g., thermal and electrical conductivity.. Alkali Characteristics.
From utedzz.blogspot.com
Periodic Table Showing Alkali Metals Alkaline Earth Metals Periodic Alkali Characteristics The key characteristic these elements share in common is that they all have one. Alkali metals have only a weak tendency to form complexes with simple lewis bases. The alkali metals are potent reductants whose chemistry is largely that of ionic compounds containing the m+ ion. The alkali metals are the elements located in group ia of the periodic table. Alkali Characteristics.
From www.slideserve.com
PPT Where are the alkali metals? PowerPoint Presentation, free Alkali Characteristics The alkali metals are potent reductants whose chemistry is largely that of ionic compounds containing the m+ ion. Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g., thermal and electrical conductivity. The key characteristic these elements share in common is that they all have one. The alkali metals are a group of elements. Alkali Characteristics.
From www.nagwa.com
Question Video Identifying the Property of Alkali Metals From a List Alkali Characteristics The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. The key characteristic these elements share in common is that they all have one. The alkali metals are potent reductants whose chemistry is largely that of ionic compounds containing the m+ ion. The alkali metals are a group. Alkali Characteristics.
From www.tes.com
Lesson Alkali Metals GCSE Edexcel 91 Teaching Resources Alkali Characteristics The alkali metals are a group of elements in the periodic table with similar properties: The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g., thermal and electrical conductivity. The alkali metals. Alkali Characteristics.
From elchoroukhost.net
Periodic Table Alkali Metals Facts Elcho Table Alkali Characteristics The key characteristic these elements share in common is that they all have one. The alkali metals are a group of elements in the periodic table with similar properties: The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. Many of the physical properties of the alkali metals. Alkali Characteristics.
From byjus.com
Alkali Metals Chemical and Physical Properties of Alkali Metals Alkali Characteristics Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g., thermal and electrical conductivity. The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. The alkali metals are the elements located in group ia of the periodic table (the first column). Alkali. Alkali Characteristics.
From knordslearning.com
Alkaline Earth Metals Periodic Table (With Images) Alkali Characteristics The alkali metals are the elements located in group ia of the periodic table (the first column). The key characteristic these elements share in common is that they all have one. Alkali metals have only a weak tendency to form complexes with simple lewis bases. The alkali metals are potent reductants whose chemistry is largely that of ionic compounds containing. Alkali Characteristics.
From www.chemistry4students.com
Chemistry 4 Students Alkali Metals (group 1 elements) Alkali Characteristics The alkali metals are potent reductants whose chemistry is largely that of ionic compounds containing the m+ ion. Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g., thermal and electrical conductivity. The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals.. Alkali Characteristics.
From www.slideserve.com
PPT THE PERIODIC TABLE PowerPoint Presentation, free download ID Alkali Characteristics The alkali metals are a group of elements in the periodic table with similar properties: The alkali metals are the elements located in group ia of the periodic table (the first column). Alkali metals have only a weak tendency to form complexes with simple lewis bases. The key characteristic these elements share in common is that they all have one.. Alkali Characteristics.
From www.vedantu.com
Alkali Metals Chemical Elements, Properties Alkali Metals Periodic Alkali Characteristics The alkali metals are potent reductants whose chemistry is largely that of ionic compounds containing the m+ ion. Alkali metals have only a weak tendency to form complexes with simple lewis bases. The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. The alkali metals are a group. Alkali Characteristics.
From ar.inspiredpencil.com
Members Of The Alkali Metals Alkali Characteristics The alkali metals are the elements located in group ia of the periodic table (the first column). Alkali metals have only a weak tendency to form complexes with simple lewis bases. The key characteristic these elements share in common is that they all have one. The alkali metals are a group of elements in the periodic table with similar properties:. Alkali Characteristics.
From www.sliderbase.com
Element Classes Presentation Chemistry Alkali Characteristics The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. The alkali metals are potent reductants whose chemistry is largely that of ionic compounds containing the m+ ion. The key characteristic these elements share in common is that they all have one. The alkali metals are the elements. Alkali Characteristics.
From www.pinterest.com
Acids, Alkalis, and the pH Scale Chemistry lessons, Chemistry Alkali Characteristics The alkali metals are the elements located in group ia of the periodic table (the first column). Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g., thermal and electrical conductivity. Alkali metals have only a weak tendency to form complexes with simple lewis bases. The key characteristic these elements share in common is. Alkali Characteristics.
From www.britannica.com
Characteristics of alkali metals Britannica Alkali Characteristics Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g., thermal and electrical conductivity. The alkali metals are the elements located in group ia of the periodic table (the first column). The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. The. Alkali Characteristics.
From www.youtube.com
Characteristics Of Noble Gas, Alkali and Alkaline Earth Metals With Alkali Characteristics The alkali metals are the elements located in group ia of the periodic table (the first column). The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. The alkali metals are potent reductants whose chemistry is largely that of ionic compounds containing the m+ ion. Many of the. Alkali Characteristics.
From www.youtube.com
Alkali Metals YouTube Alkali Characteristics Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g., thermal and electrical conductivity. The alkali metals are a group of elements in the periodic table with similar properties: The alkali metals are the elements located in group ia of the periodic table (the first column). The key characteristic these elements share in common. Alkali Characteristics.
From xlskoor.blogspot.com
Alkali Metals Chemistry Alkali Characteristics The alkali metals are the elements located in group ia of the periodic table (the first column). The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. Alkali metals have only a weak tendency to form complexes with simple lewis bases. The key characteristic these elements share in. Alkali Characteristics.
From www.differencebetween.com
Difference Between Alkali Metals and Alkaline Earth Metals Compare Alkali Characteristics The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. The alkali metals are potent reductants whose chemistry is largely that of ionic compounds containing the m+ ion. The key characteristic these elements share in common is that they all have one. Alkali metals have only a weak. Alkali Characteristics.
From www.slideserve.com
PPT Acids and Alkalis PowerPoint Presentation, free download ID3411390 Alkali Characteristics The key characteristic these elements share in common is that they all have one. The alkali metals are the elements located in group ia of the periodic table (the first column). Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g., thermal and electrical conductivity. The alkali metals exhibit many of the physical properties. Alkali Characteristics.
From www.pinterest.com
Element Infographics The Alkali Metals by Compound Interest Alkali Alkali Characteristics The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. The key characteristic these elements share in common is that they all have one. The alkali metals are the elements located in group ia of the periodic table (the first column). Many of the physical properties of the. Alkali Characteristics.
From www.slideserve.com
PPT Alkaline Earth Metals PowerPoint Presentation ID2372300 Alkali Characteristics The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g., thermal and electrical conductivity. The alkali metals are potent reductants whose chemistry is largely that of ionic compounds containing the m+ ion.. Alkali Characteristics.
From www.slideserve.com
PPT Atomic Theory PowerPoint Presentation, free download ID5435746 Alkali Characteristics The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g., thermal and electrical conductivity. The alkali metals are the elements located in group ia of the periodic table (the first column). Alkali. Alkali Characteristics.
From byjus.com
Alkaline Earth Metals General Characteristics of Oxides, Hallides Alkali Characteristics The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. The key characteristic these elements share in common is that they all have one. The alkali metals are the elements located in group ia of the periodic table (the first column). The alkali metals are a group of. Alkali Characteristics.
From www.slideserve.com
PPT Section 7.7 Group Trends for the Active Metals PowerPoint Alkali Characteristics Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g., thermal and electrical conductivity. The alkali metals are the elements located in group ia of the periodic table (the first column). Alkali metals have only a weak tendency to form complexes with simple lewis bases. The key characteristic these elements share in common is. Alkali Characteristics.
From cabinet.matttroy.net
Alkali Metals Periodic Table Located Matttroy Alkali Characteristics The key characteristic these elements share in common is that they all have one. Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g., thermal and electrical conductivity. Alkali metals have only a weak tendency to form complexes with simple lewis bases. The alkali metals are the elements located in group ia of the. Alkali Characteristics.
From elchoroukhost.net
Alkali Metals Periodic Table Location Elcho Table Alkali Characteristics Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g., thermal and electrical conductivity. The key characteristic these elements share in common is that they all have one. The alkali metals are the elements located in group ia of the periodic table (the first column). The alkali metals are a group of elements in. Alkali Characteristics.
From www.slideshare.net
ChemistryGeneral Characteristics Of Alkali Metals Alkali Characteristics Many of the physical properties of the alkali metals (table \(\pageindex{3}\).4) are typical of metals, e.g., thermal and electrical conductivity. The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. The key characteristic these elements share in common is that they all have one. The alkali metals are. Alkali Characteristics.
From www.expii.com
Alkaline Earth Metals — Overview & Properties Expii Alkali Characteristics The key characteristic these elements share in common is that they all have one. The alkali metals are potent reductants whose chemistry is largely that of ionic compounds containing the m+ ion. The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. The alkali metals are a group. Alkali Characteristics.