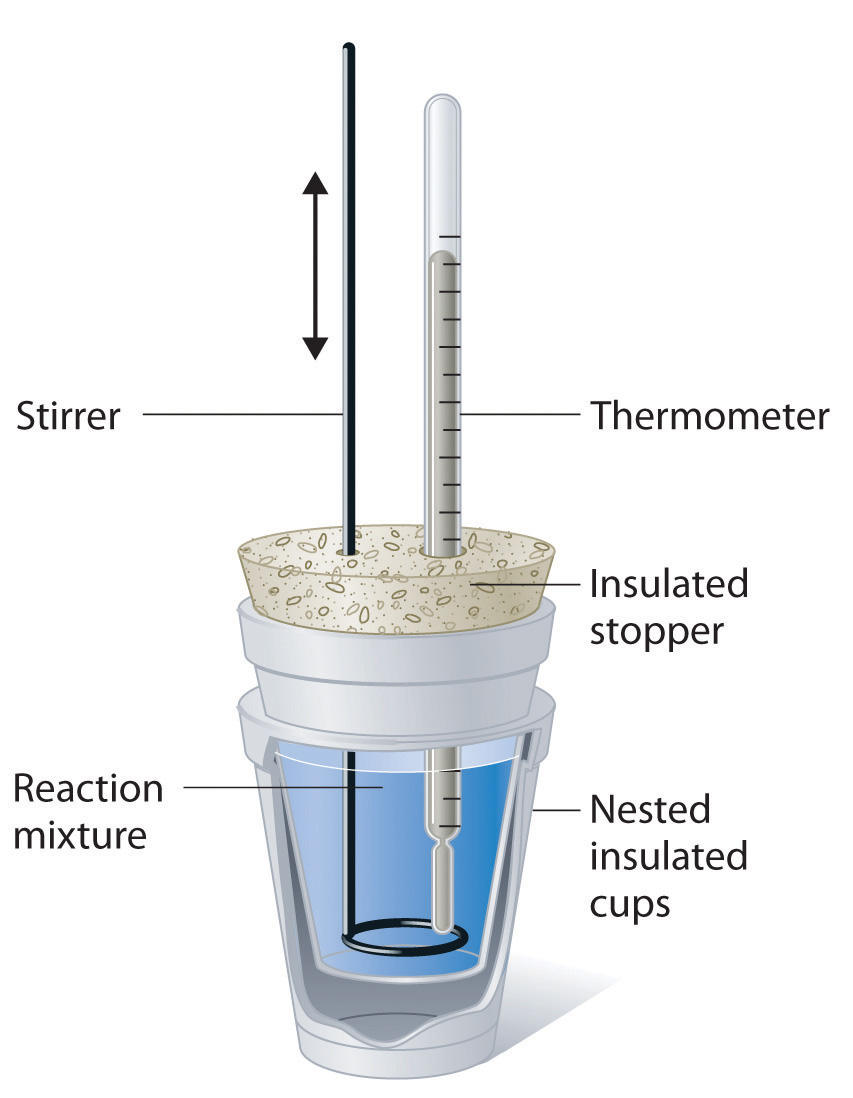
Calorimetry Experiment Example . • measure the amount of heat. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Cyclohexane fuel is burned completely in a calorimeter. Calorimetry is used to measure amounts of heat transferred to or. Below are some example calculations based on the calorimetry experiment. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. It is an insulated apparatus. For example, thermacare® uses the heat produced by the oxidation of iron powder with oxygen. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. Calorimetry is the science of measuring heat flow. As part of this lab, you will: In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known amount of. You will perform several different calorimetry experiments and use the temperature changes to calculate the specific heat of a metal, the heat of. There are 200 g of water in the. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower.
from 2012books.lardbucket.org
In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known amount of. It is an insulated apparatus. Below are some example calculations based on the calorimetry experiment. Calorimetry is used to measure amounts of heat transferred to or. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. There are 200 g of water in the. For example, thermacare® uses the heat produced by the oxidation of iron powder with oxygen. As part of this lab, you will: Calorimetry is the science of measuring heat flow.
Calorimetry
Calorimetry Experiment Example One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. You will perform several different calorimetry experiments and use the temperature changes to calculate the specific heat of a metal, the heat of. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. There are 200 g of water in the. • measure the amount of heat. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. As part of this lab, you will: Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. Below are some example calculations based on the calorimetry experiment. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known amount of. Calorimetry is used to measure amounts of heat transferred to or. It is an insulated apparatus. Cyclohexane fuel is burned completely in a calorimeter. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is the science of measuring heat flow. For example, thermacare® uses the heat produced by the oxidation of iron powder with oxygen.
From www.freeastroscience.com
Exploring Calorimetry A Journey into Heat Transfer Measurement Calorimetry Experiment Example One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. For example, thermacare® uses the heat produced by the oxidation of iron powder with oxygen. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. Below are. Calorimetry Experiment Example.
From dxojkdaez.blob.core.windows.net
Calorimetry Physics Examples at Jeremy Wetmore blog Calorimetry Experiment Example For example, thermacare® uses the heat produced by the oxidation of iron powder with oxygen. Calorimetry is used to measure amounts of heat transferred to or. Below are some example calculations based on the calorimetry experiment. Cyclohexane fuel is burned completely in a calorimeter. It is an insulated apparatus. Simple calorimetry experiments can be used to calculate the heat energy. Calorimetry Experiment Example.
From www.youtube.com
050 Calorimetry YouTube Calorimetry Experiment Example As part of this lab, you will: One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. You will perform several different calorimetry experiments and use the. Calorimetry Experiment Example.
From www.youtube.com
CALORIMETRY EXAMPLE 3 YouTube Calorimetry Experiment Example • measure the amount of heat. Calorimetry is the science of measuring heat flow. Cyclohexane fuel is burned completely in a calorimeter. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. Heat is defined as thermal energy flowing from an object at a higher temperature to one at. Calorimetry Experiment Example.
From kaffee.50webs.com
Lab Calorimetry Calorimetry Experiment Example As part of this lab, you will: There are 200 g of water in the. For example, thermacare® uses the heat produced by the oxidation of iron powder with oxygen. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. You will perform several different calorimetry experiments and use the temperature. Calorimetry Experiment Example.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimetry Experiment Example Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Below are some example calculations based on the calorimetry experiment. You will perform several different calorimetry experiments and use the temperature changes to calculate the specific heat of a metal, the heat of. • measure the amount of. Calorimetry Experiment Example.
From klarxnzah.blob.core.windows.net
Calorimetry Experiment With Different Metals at David Lytton blog Calorimetry Experiment Example There are 200 g of water in the. You will perform several different calorimetry experiments and use the temperature changes to calculate the specific heat of a metal, the heat of. Cyclohexane fuel is burned completely in a calorimeter. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as. Calorimetry Experiment Example.
From exonwzknq.blob.core.windows.net
Calorimetry Experiment Gcse Chemistry at Sanders blog Calorimetry Experiment Example You will perform several different calorimetry experiments and use the temperature changes to calculate the specific heat of a metal, the heat of. There are 200 g of water in the. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. • measure the amount of heat. Below. Calorimetry Experiment Example.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Calorimetry Experiment Example Below are some example calculations based on the calorimetry experiment. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. You will perform several different calorimetry experiments and use the temperature changes to calculate the specific heat of a metal, the heat of. Simple calorimetry experiments can be used to calculate. Calorimetry Experiment Example.
From 2012books.lardbucket.org
Calorimetry Calorimetry Experiment Example You will perform several different calorimetry experiments and use the temperature changes to calculate the specific heat of a metal, the heat of. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. • measure the amount of heat. As part of this lab, you will: Calorimetry is used to measure. Calorimetry Experiment Example.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Calorimetry Experiment Example You will perform several different calorimetry experiments and use the temperature changes to calculate the specific heat of a metal, the heat of. • measure the amount of heat. There are 200 g of water in the. Calorimetry is used to measure amounts of heat transferred to or. Calorimetry is the study of heat transferred in a chemical reaction, and. Calorimetry Experiment Example.
From www.youtube.com
Heat of Reaction (Calorimetry) Experiment YouTube Calorimetry Experiment Example It is an insulated apparatus. Cyclohexane fuel is burned completely in a calorimeter. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. • measure the amount. Calorimetry Experiment Example.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Experiment Example Calorimetry is the science of measuring heat flow. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. Cyclohexane fuel is burned completely in a calorimeter. Below are some example calculations based on the calorimetry experiment. It is an insulated apparatus. There are 200 g of water in the.. Calorimetry Experiment Example.
From www.slideserve.com
PPT Chapter 12 “Heat in Chemical Reactions” PowerPoint Presentation Calorimetry Experiment Example It is an insulated apparatus. Below are some example calculations based on the calorimetry experiment. • measure the amount of heat. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used. Calorimetry Experiment Example.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimetry Experiment Example Calorimetry is used to measure amounts of heat transferred to or. As part of this lab, you will: Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. It is an insulated apparatus. There are 200 g of water in the. You will perform several different calorimetry experiments and. Calorimetry Experiment Example.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimetry Experiment Example One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. • measure the amount of heat. For example, thermacare® uses the heat produced by the oxidation of iron powder with oxygen. You will perform several different calorimetry experiments and use the temperature changes to calculate the specific heat. Calorimetry Experiment Example.
From www.education.com
Calorimetry Bomb Calorimeter Experiment Calorimetry Experiment Example Calorimetry is the science of measuring heat flow. As part of this lab, you will: Cyclohexane fuel is burned completely in a calorimeter. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. Calorimetry is used to measure amounts of heat transferred to or. There are 200 g of. Calorimetry Experiment Example.
From exonwzknq.blob.core.windows.net
Calorimetry Experiment Gcse Chemistry at Sanders blog Calorimetry Experiment Example There are 200 g of water in the. Below are some example calculations based on the calorimetry experiment. It is an insulated apparatus. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. One technique we can use to measure the amount of heat involved in a chemical or physical process. Calorimetry Experiment Example.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Calorimetry Experiment Example Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. As part of this lab, you will: Cyclohexane fuel is burned completely in a calorimeter. Below are some example calculations based on the calorimetry experiment. Calorimetry is the science of measuring heat flow. It is an insulated apparatus. You. Calorimetry Experiment Example.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 3.2 Describe Simple Calorimetry Experiments for Calorimetry Experiment Example One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known amount of. Below are some example calculations based on the calorimetry. Calorimetry Experiment Example.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Experiment Example • measure the amount of heat. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is the science of measuring heat flow. It is an insulated apparatus. In this experiment you will heat a known mass of a metal to a known temperature and then transfer. Calorimetry Experiment Example.
From www.pathwaystochemistry.com
Calorimetry Pathways to Chemistry Calorimetry Experiment Example Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. You will perform several different calorimetry experiments and use the temperature changes to calculate the specific heat of a. Calorimetry Experiment Example.
From www.youtube.com
Calorimetry Experiment YouTube Calorimetry Experiment Example Calorimetry is the science of measuring heat flow. It is an insulated apparatus. For example, thermacare® uses the heat produced by the oxidation of iron powder with oxygen. Below are some example calculations based on the calorimetry experiment. You will perform several different calorimetry experiments and use the temperature changes to calculate the specific heat of a metal, the heat. Calorimetry Experiment Example.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Calorimetry Experiment Example Calorimetry is the science of measuring heat flow. There are 200 g of water in the. Below are some example calculations based on the calorimetry experiment. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. As part of this lab, you will: For example, thermacare® uses the. Calorimetry Experiment Example.
From www.savemyexams.com
Calorimetry Experiments SL IB Chemistry Revision Notes 2025 Save My Calorimetry Experiment Example Cyclohexane fuel is burned completely in a calorimeter. There are 200 g of water in the. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. •. Calorimetry Experiment Example.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimetry Experiment Example It is an insulated apparatus. For example, thermacare® uses the heat produced by the oxidation of iron powder with oxygen. Below are some example calculations based on the calorimetry experiment. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. Calorimetry is the science of measuring heat flow. In. Calorimetry Experiment Example.
From klarxnzah.blob.core.windows.net
Calorimetry Experiment With Different Metals at David Lytton blog Calorimetry Experiment Example For example, thermacare® uses the heat produced by the oxidation of iron powder with oxygen. Below are some example calculations based on the calorimetry experiment. Calorimetry is used to measure amounts of heat transferred to or. There are 200 g of water in the. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the. Calorimetry Experiment Example.
From courses.lumenlearning.com
Calorimetry Chemistry Calorimetry Experiment Example • measure the amount of heat. Below are some example calculations based on the calorimetry experiment. For example, thermacare® uses the heat produced by the oxidation of iron powder with oxygen. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. In this experiment you will heat a. Calorimetry Experiment Example.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Calorimetry Experiment Example Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. Below are some example calculations based on the calorimetry experiment. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known amount of.. Calorimetry Experiment Example.
From www.youtube.com
Calorimetry Part II Lab Version) YouTube Calorimetry Experiment Example There are 200 g of water in the. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. Cyclohexane fuel is burned completely in a calorimeter. Calorimetry is the science of measuring heat flow. • measure the amount of heat. In this experiment you will heat a known mass of a. Calorimetry Experiment Example.
From www.youtube.com
Physics 9.09g Calorimetry Example 2 YouTube Calorimetry Experiment Example Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. Below are some example calculations based on the calorimetry experiment. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. As part of this lab, you will: • measure the. Calorimetry Experiment Example.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Calorimetry Experiment Example Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. There are 200 g of water in the. Cyclohexane fuel is burned completely in a calorimeter. Simple calorimetry experiments. Calorimetry Experiment Example.
From www.youtube.com
Unit 8 Food Calorimetry Lab YouTube Calorimetry Experiment Example As part of this lab, you will: You will perform several different calorimetry experiments and use the temperature changes to calculate the specific heat of a metal, the heat of. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. • measure the amount of heat. Simple calorimetry. Calorimetry Experiment Example.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimetry Experiment Example • measure the amount of heat. Calorimetry is used to measure amounts of heat transferred to or. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. As part of this lab, you will: Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is. Calorimetry Experiment Example.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Calorimetry Experiment Example You will perform several different calorimetry experiments and use the temperature changes to calculate the specific heat of a metal, the heat of. It is an insulated apparatus. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. Calorimetry is the study of heat transferred in a chemical reaction, and a. Calorimetry Experiment Example.