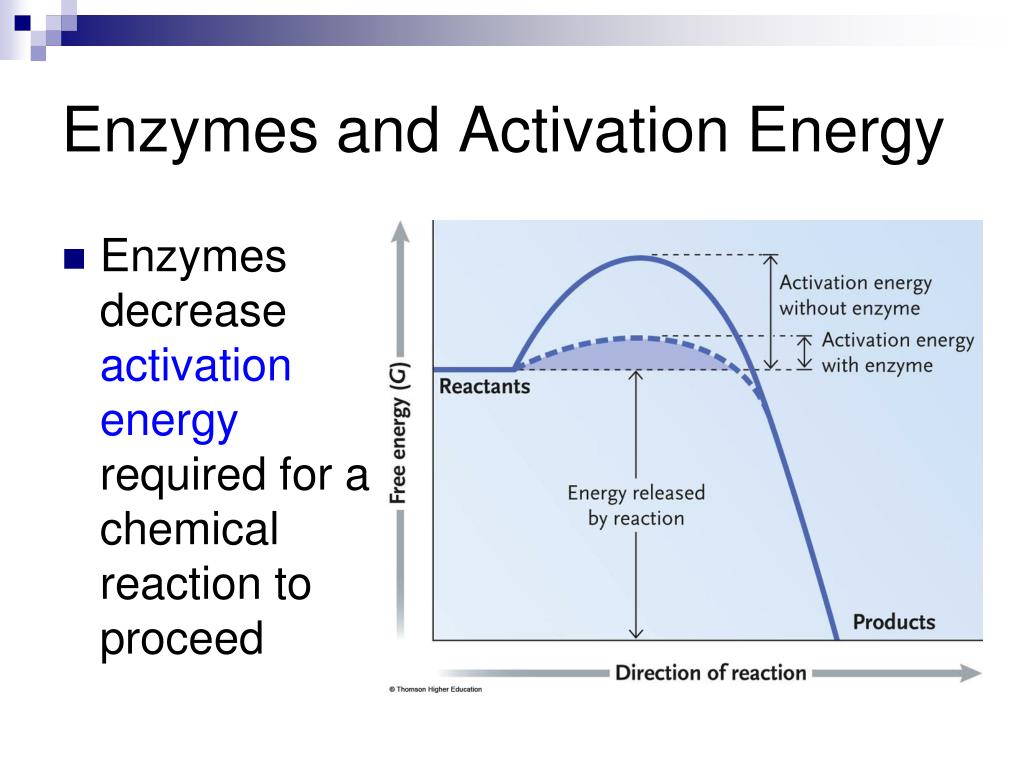
Do Enzymes Decrease Delta G . Although the sign of δg (negative or positive) determines the direction that the reaction will go spontaneously, the magnitude of δg does not. enzymes do not affect δg or δgo between the substrate and the product. The change in free energy can be calculated for any system that undergoes a. Enzymes do affect the activation energy. every chemical reaction involves a change in free energy, called delta g (∆g). Discuss enzyme regulation by various factors. A substance that helps a. how can enzymes reduce the activation energy of every forward reaction but still not affect the $\delta h$? explain how enzymes function as molecular catalysts. the small molecules that are the reactants and products of a reaction are called metabolites, and the catalysts that.
from www.slideserve.com
every chemical reaction involves a change in free energy, called delta g (∆g). the small molecules that are the reactants and products of a reaction are called metabolites, and the catalysts that. A substance that helps a. Discuss enzyme regulation by various factors. Although the sign of δg (negative or positive) determines the direction that the reaction will go spontaneously, the magnitude of δg does not. enzymes do not affect δg or δgo between the substrate and the product. The change in free energy can be calculated for any system that undergoes a. explain how enzymes function as molecular catalysts. how can enzymes reduce the activation energy of every forward reaction but still not affect the $\delta h$? Enzymes do affect the activation energy.
PPT Energy, Enzymes, and Biological Reactions PowerPoint Presentation
Do Enzymes Decrease Delta G every chemical reaction involves a change in free energy, called delta g (∆g). Although the sign of δg (negative or positive) determines the direction that the reaction will go spontaneously, the magnitude of δg does not. the small molecules that are the reactants and products of a reaction are called metabolites, and the catalysts that. The change in free energy can be calculated for any system that undergoes a. Discuss enzyme regulation by various factors. enzymes do not affect δg or δgo between the substrate and the product. how can enzymes reduce the activation energy of every forward reaction but still not affect the $\delta h$? explain how enzymes function as molecular catalysts. A substance that helps a. every chemical reaction involves a change in free energy, called delta g (∆g). Enzymes do affect the activation energy.
From thebiologs.blogspot.com
The BioLogs Ezymes CSEC Do Enzymes Decrease Delta G enzymes do not affect δg or δgo between the substrate and the product. every chemical reaction involves a change in free energy, called delta g (∆g). the small molecules that are the reactants and products of a reaction are called metabolites, and the catalysts that. The change in free energy can be calculated for any system that. Do Enzymes Decrease Delta G.
From www2.nau.edu
Enzymes and Reaction Rates Do Enzymes Decrease Delta G enzymes do not affect δg or δgo between the substrate and the product. how can enzymes reduce the activation energy of every forward reaction but still not affect the $\delta h$? The change in free energy can be calculated for any system that undergoes a. the small molecules that are the reactants and products of a reaction. Do Enzymes Decrease Delta G.
From biology4ibdp.weebly.com
2.5 Enzymes BIOLOGY4IBDP Do Enzymes Decrease Delta G enzymes do not affect δg or δgo between the substrate and the product. A substance that helps a. how can enzymes reduce the activation energy of every forward reaction but still not affect the $\delta h$? every chemical reaction involves a change in free energy, called delta g (∆g). The change in free energy can be calculated. Do Enzymes Decrease Delta G.
From www.onlinebiologynotes.com
Enzymes Properties and Mechanism of enzyme action Online Biology Notes Do Enzymes Decrease Delta G Although the sign of δg (negative or positive) determines the direction that the reaction will go spontaneously, the magnitude of δg does not. A substance that helps a. every chemical reaction involves a change in free energy, called delta g (∆g). Enzymes do affect the activation energy. how can enzymes reduce the activation energy of every forward reaction. Do Enzymes Decrease Delta G.
From www.chegg.com
Solved Enzymes lower the delta G of the reaction. True False Do Enzymes Decrease Delta G Although the sign of δg (negative or positive) determines the direction that the reaction will go spontaneously, the magnitude of δg does not. every chemical reaction involves a change in free energy, called delta g (∆g). the small molecules that are the reactants and products of a reaction are called metabolites, and the catalysts that. enzymes do. Do Enzymes Decrease Delta G.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry ΔG; thermodynamically Do Enzymes Decrease Delta G A substance that helps a. explain how enzymes function as molecular catalysts. enzymes do not affect δg or δgo between the substrate and the product. Although the sign of δg (negative or positive) determines the direction that the reaction will go spontaneously, the magnitude of δg does not. the small molecules that are the reactants and products. Do Enzymes Decrease Delta G.
From chemistrytalk.org
Enzymes Function and Types ChemTalk Do Enzymes Decrease Delta G Although the sign of δg (negative or positive) determines the direction that the reaction will go spontaneously, the magnitude of δg does not. Enzymes do affect the activation energy. the small molecules that are the reactants and products of a reaction are called metabolites, and the catalysts that. how can enzymes reduce the activation energy of every forward. Do Enzymes Decrease Delta G.
From materialcampuscarolyn.z21.web.core.windows.net
Enzymes In Biology Explained Do Enzymes Decrease Delta G The change in free energy can be calculated for any system that undergoes a. explain how enzymes function as molecular catalysts. the small molecules that are the reactants and products of a reaction are called metabolites, and the catalysts that. how can enzymes reduce the activation energy of every forward reaction but still not affect the $\delta. Do Enzymes Decrease Delta G.
From www.slideserve.com
PPT EnZyMeS PowerPoint Presentation, free download ID2065739 Do Enzymes Decrease Delta G the small molecules that are the reactants and products of a reaction are called metabolites, and the catalysts that. A substance that helps a. Discuss enzyme regulation by various factors. The change in free energy can be calculated for any system that undergoes a. Although the sign of δg (negative or positive) determines the direction that the reaction will. Do Enzymes Decrease Delta G.
From www.slideserve.com
PPT Energy, Enzymes, and Biological Reactions PowerPoint Presentation Do Enzymes Decrease Delta G how can enzymes reduce the activation energy of every forward reaction but still not affect the $\delta h$? every chemical reaction involves a change in free energy, called delta g (∆g). A substance that helps a. the small molecules that are the reactants and products of a reaction are called metabolites, and the catalysts that. Discuss enzyme. Do Enzymes Decrease Delta G.
From www.youtube.com
Enzyme Basics & How Do They Lower Activation Energy? YouTube Do Enzymes Decrease Delta G enzymes do not affect δg or δgo between the substrate and the product. how can enzymes reduce the activation energy of every forward reaction but still not affect the $\delta h$? explain how enzymes function as molecular catalysts. A substance that helps a. Discuss enzyme regulation by various factors. the small molecules that are the reactants. Do Enzymes Decrease Delta G.
From www.slideserve.com
PPT Enzymes PowerPoint Presentation, free download ID1940469 Do Enzymes Decrease Delta G how can enzymes reduce the activation energy of every forward reaction but still not affect the $\delta h$? Discuss enzyme regulation by various factors. Enzymes do affect the activation energy. enzymes do not affect δg or δgo between the substrate and the product. every chemical reaction involves a change in free energy, called delta g (∆g). . Do Enzymes Decrease Delta G.
From exoaynyrx.blob.core.windows.net
Do Enzymes Bind To Receptors at Gregory Boswell blog Do Enzymes Decrease Delta G every chemical reaction involves a change in free energy, called delta g (∆g). enzymes do not affect δg or δgo between the substrate and the product. A substance that helps a. the small molecules that are the reactants and products of a reaction are called metabolites, and the catalysts that. how can enzymes reduce the activation. Do Enzymes Decrease Delta G.
From ibiologia.com
Enzymes Definition, Classification & Functions Do Enzymes Decrease Delta G the small molecules that are the reactants and products of a reaction are called metabolites, and the catalysts that. enzymes do not affect δg or δgo between the substrate and the product. every chemical reaction involves a change in free energy, called delta g (∆g). Enzymes do affect the activation energy. A substance that helps a. . Do Enzymes Decrease Delta G.
From exojcetvn.blob.core.windows.net
Enzymes Is Produced By at Patrick Rogers blog Do Enzymes Decrease Delta G Discuss enzyme regulation by various factors. explain how enzymes function as molecular catalysts. enzymes do not affect δg or δgo between the substrate and the product. The change in free energy can be calculated for any system that undergoes a. the small molecules that are the reactants and products of a reaction are called metabolites, and the. Do Enzymes Decrease Delta G.
From exolhjqte.blob.core.windows.net
Enzymes Chemical Pathways at Norma Reynolds blog Do Enzymes Decrease Delta G how can enzymes reduce the activation energy of every forward reaction but still not affect the $\delta h$? explain how enzymes function as molecular catalysts. Enzymes do affect the activation energy. Discuss enzyme regulation by various factors. The change in free energy can be calculated for any system that undergoes a. the small molecules that are the. Do Enzymes Decrease Delta G.
From www.slideserve.com
PPT Enzymes PowerPoint Presentation, free download ID1936659 Do Enzymes Decrease Delta G how can enzymes reduce the activation energy of every forward reaction but still not affect the $\delta h$? Discuss enzyme regulation by various factors. Although the sign of δg (negative or positive) determines the direction that the reaction will go spontaneously, the magnitude of δg does not. explain how enzymes function as molecular catalysts. Enzymes do affect the. Do Enzymes Decrease Delta G.
From www.slideserve.com
PPT Enzyme Rate Enhancement PowerPoint Presentation, free download Do Enzymes Decrease Delta G explain how enzymes function as molecular catalysts. The change in free energy can be calculated for any system that undergoes a. every chemical reaction involves a change in free energy, called delta g (∆g). how can enzymes reduce the activation energy of every forward reaction but still not affect the $\delta h$? Although the sign of δg. Do Enzymes Decrease Delta G.
From ditki.com
Biochemistry Glossary Enzyme Overview ditki medical & biological Do Enzymes Decrease Delta G how can enzymes reduce the activation energy of every forward reaction but still not affect the $\delta h$? A substance that helps a. Although the sign of δg (negative or positive) determines the direction that the reaction will go spontaneously, the magnitude of δg does not. the small molecules that are the reactants and products of a reaction. Do Enzymes Decrease Delta G.
From circuitwiringtray.z13.web.core.windows.net
Glycolysis Cycle Diagram With Enzymes Do Enzymes Decrease Delta G Enzymes do affect the activation energy. how can enzymes reduce the activation energy of every forward reaction but still not affect the $\delta h$? Discuss enzyme regulation by various factors. explain how enzymes function as molecular catalysts. Although the sign of δg (negative or positive) determines the direction that the reaction will go spontaneously, the magnitude of δg. Do Enzymes Decrease Delta G.
From www.researchgate.net
Enzymes Decrease the Activation Energy Download Scientific Diagram Do Enzymes Decrease Delta G how can enzymes reduce the activation energy of every forward reaction but still not affect the $\delta h$? Although the sign of δg (negative or positive) determines the direction that the reaction will go spontaneously, the magnitude of δg does not. The change in free energy can be calculated for any system that undergoes a. Discuss enzyme regulation by. Do Enzymes Decrease Delta G.
From www.slideserve.com
PPT Concept 8.4 Enzymes speed up metabolic reactions by lowering Do Enzymes Decrease Delta G Discuss enzyme regulation by various factors. The change in free energy can be calculated for any system that undergoes a. explain how enzymes function as molecular catalysts. every chemical reaction involves a change in free energy, called delta g (∆g). Although the sign of δg (negative or positive) determines the direction that the reaction will go spontaneously, the. Do Enzymes Decrease Delta G.
From jumpstarterdiscount.blogspot.com
The Diagram Below Represents A Spontaneous Reaction δg Wiring Diagram Do Enzymes Decrease Delta G explain how enzymes function as molecular catalysts. every chemical reaction involves a change in free energy, called delta g (∆g). how can enzymes reduce the activation energy of every forward reaction but still not affect the $\delta h$? The change in free energy can be calculated for any system that undergoes a. Discuss enzyme regulation by various. Do Enzymes Decrease Delta G.
From www.biologynotes.in
Enzyme Specificity Definition, Types, Examples and Importance Do Enzymes Decrease Delta G enzymes do not affect δg or δgo between the substrate and the product. the small molecules that are the reactants and products of a reaction are called metabolites, and the catalysts that. Although the sign of δg (negative or positive) determines the direction that the reaction will go spontaneously, the magnitude of δg does not. The change in. Do Enzymes Decrease Delta G.
From www.chegg.com
Solved Which of the following statements about enzymes is Do Enzymes Decrease Delta G Discuss enzyme regulation by various factors. A substance that helps a. Enzymes do affect the activation energy. every chemical reaction involves a change in free energy, called delta g (∆g). Although the sign of δg (negative or positive) determines the direction that the reaction will go spontaneously, the magnitude of δg does not. how can enzymes reduce the. Do Enzymes Decrease Delta G.
From www.slideserve.com
PPT HOW ENZYMES WORK PowerPoint Presentation, free download ID6954410 Do Enzymes Decrease Delta G the small molecules that are the reactants and products of a reaction are called metabolites, and the catalysts that. Enzymes do affect the activation energy. explain how enzymes function as molecular catalysts. Although the sign of δg (negative or positive) determines the direction that the reaction will go spontaneously, the magnitude of δg does not. every chemical. Do Enzymes Decrease Delta G.
From www.slideserve.com
PPT HOW ENZYMES WORK PowerPoint Presentation, free download ID6954410 Do Enzymes Decrease Delta G every chemical reaction involves a change in free energy, called delta g (∆g). Discuss enzyme regulation by various factors. Although the sign of δg (negative or positive) determines the direction that the reaction will go spontaneously, the magnitude of δg does not. enzymes do not affect δg or δgo between the substrate and the product. A substance that. Do Enzymes Decrease Delta G.
From www.youtube.com
"Enzymes Lower Activation Energy” for GCSE Biology and A Level Biology Do Enzymes Decrease Delta G the small molecules that are the reactants and products of a reaction are called metabolites, and the catalysts that. A substance that helps a. Discuss enzyme regulation by various factors. The change in free energy can be calculated for any system that undergoes a. how can enzymes reduce the activation energy of every forward reaction but still not. Do Enzymes Decrease Delta G.
From www.slideserve.com
PPT Chemical reactions and enzymes PowerPoint Presentation, free Do Enzymes Decrease Delta G how can enzymes reduce the activation energy of every forward reaction but still not affect the $\delta h$? The change in free energy can be calculated for any system that undergoes a. Discuss enzyme regulation by various factors. enzymes do not affect δg or δgo between the substrate and the product. every chemical reaction involves a change. Do Enzymes Decrease Delta G.
From www.numerade.com
SOLVED Approximately how much do the enzymes a. staphylococcal Do Enzymes Decrease Delta G every chemical reaction involves a change in free energy, called delta g (∆g). Discuss enzyme regulation by various factors. Although the sign of δg (negative or positive) determines the direction that the reaction will go spontaneously, the magnitude of δg does not. the small molecules that are the reactants and products of a reaction are called metabolites, and. Do Enzymes Decrease Delta G.
From www.slideserve.com
PPT Chapter 6 Enzymes PowerPoint Presentation, free download ID5143485 Do Enzymes Decrease Delta G explain how enzymes function as molecular catalysts. The change in free energy can be calculated for any system that undergoes a. every chemical reaction involves a change in free energy, called delta g (∆g). Although the sign of δg (negative or positive) determines the direction that the reaction will go spontaneously, the magnitude of δg does not. Discuss. Do Enzymes Decrease Delta G.
From www.slideserve.com
PPT Chapter 6 Basic Concepts of Enzyme Action PowerPoint Do Enzymes Decrease Delta G enzymes do not affect δg or δgo between the substrate and the product. A substance that helps a. every chemical reaction involves a change in free energy, called delta g (∆g). Enzymes do affect the activation energy. how can enzymes reduce the activation energy of every forward reaction but still not affect the $\delta h$? Although the. Do Enzymes Decrease Delta G.
From www.biomol.com
Guide to Enzyme Unit Definitions and Assay Design Biomol Blog Do Enzymes Decrease Delta G how can enzymes reduce the activation energy of every forward reaction but still not affect the $\delta h$? Although the sign of δg (negative or positive) determines the direction that the reaction will go spontaneously, the magnitude of δg does not. Enzymes do affect the activation energy. explain how enzymes function as molecular catalysts. Discuss enzyme regulation by. Do Enzymes Decrease Delta G.
From www.genome.gov
Enzyme Do Enzymes Decrease Delta G explain how enzymes function as molecular catalysts. every chemical reaction involves a change in free energy, called delta g (∆g). enzymes do not affect δg or δgo between the substrate and the product. Enzymes do affect the activation energy. how can enzymes reduce the activation energy of every forward reaction but still not affect the $\delta. Do Enzymes Decrease Delta G.
From www.slideserve.com
PPT Enzymes PowerPoint Presentation, free download ID650440 Do Enzymes Decrease Delta G A substance that helps a. Enzymes do affect the activation energy. how can enzymes reduce the activation energy of every forward reaction but still not affect the $\delta h$? Discuss enzyme regulation by various factors. Although the sign of δg (negative or positive) determines the direction that the reaction will go spontaneously, the magnitude of δg does not. . Do Enzymes Decrease Delta G.