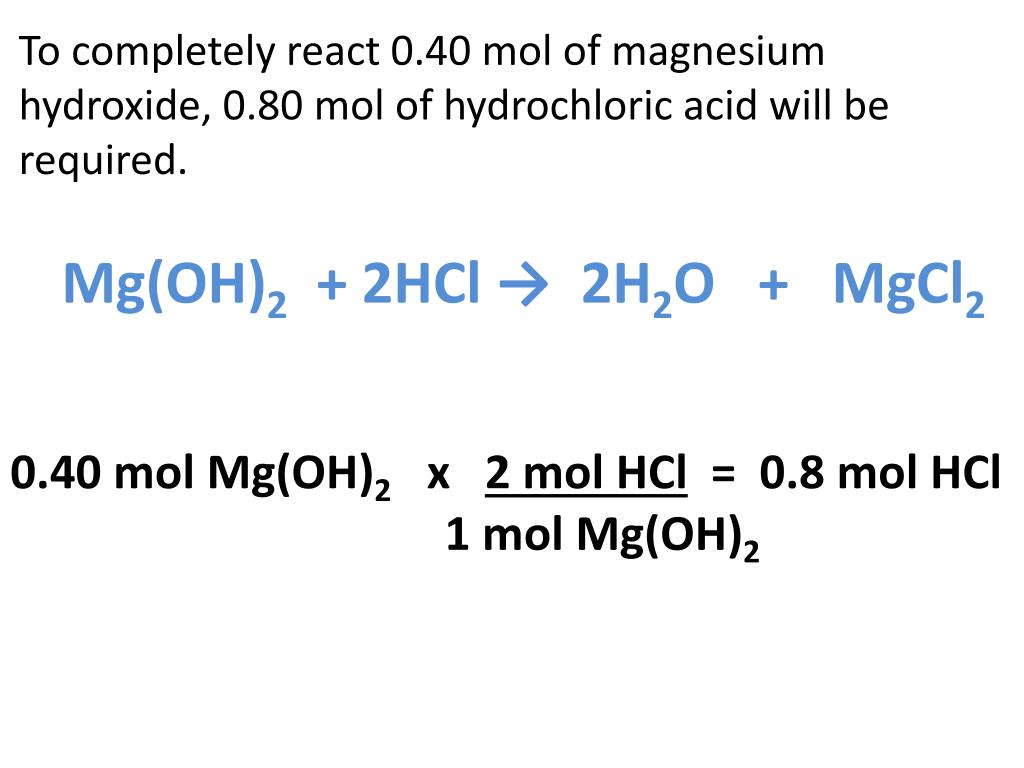Dissociation Magnesium Hydroxide Formula . Magnesium hydroxide is an ionic compound. Magnesium hydroxide is an ionic compound, so when it dissolves it dissociates. Thus, magnesium hydroxide is an electrolyte. When mg (oh)₂ dissolves in. (a) adding a common ion, mg 2+, will increase the concentration of this ion and shift the solubility equilibrium to the left, decreasing the. It consists of mg²⁺ ions and oh⁻ ions. In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. Its formula is mg (oh)₂. Which chemical equation shows the dissociation of magnesium hydroxide?
from www.slideserve.com
Magnesium hydroxide is an ionic compound. Thus, magnesium hydroxide is an electrolyte. Magnesium hydroxide is an ionic compound, so when it dissolves it dissociates. It consists of mg²⁺ ions and oh⁻ ions. (a) adding a common ion, mg 2+, will increase the concentration of this ion and shift the solubility equilibrium to the left, decreasing the. When mg (oh)₂ dissolves in. Its formula is mg (oh)₂. Which chemical equation shows the dissociation of magnesium hydroxide? In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is.
PPT Mg(OH) 2 + 2HCl → 2H 2 O + MgCl 2 PowerPoint Presentation, free
Dissociation Magnesium Hydroxide Formula (a) adding a common ion, mg 2+, will increase the concentration of this ion and shift the solubility equilibrium to the left, decreasing the. Magnesium hydroxide is an ionic compound, so when it dissolves it dissociates. In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. It consists of mg²⁺ ions and oh⁻ ions. When mg (oh)₂ dissolves in. Magnesium hydroxide is an ionic compound. (a) adding a common ion, mg 2+, will increase the concentration of this ion and shift the solubility equilibrium to the left, decreasing the. Thus, magnesium hydroxide is an electrolyte. Which chemical equation shows the dissociation of magnesium hydroxide? Its formula is mg (oh)₂.
From www.numerade.com
SOLVED Enter a net ionic equation for the reaction between nitric acid Dissociation Magnesium Hydroxide Formula In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. When mg (oh)₂ dissolves in. Magnesium hydroxide is an ionic compound. Its formula is mg (oh)₂. Thus, magnesium hydroxide is an electrolyte. It consists of mg²⁺ ions and oh⁻ ions. (a) adding a common ion, mg 2+, will increase the. Dissociation Magnesium Hydroxide Formula.
From animalia-life.club
Magnesium Hydroxide Formula Dissociation Magnesium Hydroxide Formula In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. Which chemical equation shows the dissociation of magnesium hydroxide? Magnesium hydroxide is an ionic compound. Magnesium hydroxide is an ionic compound, so when it dissolves it dissociates. Its formula is mg (oh)₂. (a) adding a common ion, mg 2+, will. Dissociation Magnesium Hydroxide Formula.
From www.slideserve.com
PPT Acid Base Class 4 PowerPoint Presentation, free download ID Dissociation Magnesium Hydroxide Formula In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. It consists of mg²⁺ ions and oh⁻ ions. Which chemical equation shows the dissociation of magnesium hydroxide? Magnesium hydroxide is an ionic compound. (a) adding a common ion, mg 2+, will increase the concentration of this ion and shift the. Dissociation Magnesium Hydroxide Formula.
From lilynewsbranch.blogspot.com
Which Chemical Equation Shows the Dissociation of Magnesium Hydroxide Dissociation Magnesium Hydroxide Formula Magnesium hydroxide is an ionic compound, so when it dissolves it dissociates. Magnesium hydroxide is an ionic compound. In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. It consists of mg²⁺ ions and oh⁻ ions. (a) adding a common ion, mg 2+, will increase the concentration of this ion. Dissociation Magnesium Hydroxide Formula.
From learnaboutpharma.com
Dissociation constant Definition, Explanation, Formula, Example, and Dissociation Magnesium Hydroxide Formula When mg (oh)₂ dissolves in. It consists of mg²⁺ ions and oh⁻ ions. Magnesium hydroxide is an ionic compound. Thus, magnesium hydroxide is an electrolyte. In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. Which chemical equation shows the dissociation of magnesium hydroxide? (a) adding a common ion, mg. Dissociation Magnesium Hydroxide Formula.
From www.numerade.com
SOLVEDFor the dissolution of magnesium hydroxide in water (a) Write a Dissociation Magnesium Hydroxide Formula Which chemical equation shows the dissociation of magnesium hydroxide? (a) adding a common ion, mg 2+, will increase the concentration of this ion and shift the solubility equilibrium to the left, decreasing the. Thus, magnesium hydroxide is an electrolyte. Magnesium hydroxide is an ionic compound. When mg (oh)₂ dissolves in. It consists of mg²⁺ ions and oh⁻ ions. In this. Dissociation Magnesium Hydroxide Formula.
From byjus.com
Write down the formulae ofiv Magnesium hydroxide Dissociation Magnesium Hydroxide Formula (a) adding a common ion, mg 2+, will increase the concentration of this ion and shift the solubility equilibrium to the left, decreasing the. In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. Magnesium hydroxide is an ionic compound. Thus, magnesium hydroxide is an electrolyte. When mg (oh)₂ dissolves. Dissociation Magnesium Hydroxide Formula.
From www.youtube.com
How to write the formula for magnesium hydroxide YouTube Dissociation Magnesium Hydroxide Formula Its formula is mg (oh)₂. Thus, magnesium hydroxide is an electrolyte. Magnesium hydroxide is an ionic compound. It consists of mg²⁺ ions and oh⁻ ions. (a) adding a common ion, mg 2+, will increase the concentration of this ion and shift the solubility equilibrium to the left, decreasing the. Magnesium hydroxide is an ionic compound, so when it dissolves it. Dissociation Magnesium Hydroxide Formula.
From www.numerade.com
SOLVED Write the equation for the dissociation of solid calcium Dissociation Magnesium Hydroxide Formula Magnesium hydroxide is an ionic compound. (a) adding a common ion, mg 2+, will increase the concentration of this ion and shift the solubility equilibrium to the left, decreasing the. Its formula is mg (oh)₂. In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. It consists of mg²⁺ ions. Dissociation Magnesium Hydroxide Formula.
From www.toppr.com
5. Show the dissociation of the following compounds on dissolving in Dissociation Magnesium Hydroxide Formula Thus, magnesium hydroxide is an electrolyte. Which chemical equation shows the dissociation of magnesium hydroxide? (a) adding a common ion, mg 2+, will increase the concentration of this ion and shift the solubility equilibrium to the left, decreasing the. It consists of mg²⁺ ions and oh⁻ ions. Magnesium hydroxide is an ionic compound, so when it dissolves it dissociates. Its. Dissociation Magnesium Hydroxide Formula.
From www.numerade.com
SOLVED Solid magnesium hydroxide into gaseous water and Dissociation Magnesium Hydroxide Formula Magnesium hydroxide is an ionic compound, so when it dissolves it dissociates. Magnesium hydroxide is an ionic compound. When mg (oh)₂ dissolves in. In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. (a) adding a common ion, mg 2+, will increase the concentration of this ion and shift the. Dissociation Magnesium Hydroxide Formula.
From brainly.in
the molecular formula of magnisium hydroxide by criss cross method Dissociation Magnesium Hydroxide Formula When mg (oh)₂ dissolves in. Its formula is mg (oh)₂. In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. (a) adding a common ion, mg 2+, will increase the concentration of this ion and shift the solubility equilibrium to the left, decreasing the. It consists of mg²⁺ ions and. Dissociation Magnesium Hydroxide Formula.
From ar.inspiredpencil.com
Magnesium Hydroxide Structure Dissociation Magnesium Hydroxide Formula It consists of mg²⁺ ions and oh⁻ ions. (a) adding a common ion, mg 2+, will increase the concentration of this ion and shift the solubility equilibrium to the left, decreasing the. Magnesium hydroxide is an ionic compound. Magnesium hydroxide is an ionic compound, so when it dissolves it dissociates. Thus, magnesium hydroxide is an electrolyte. Its formula is mg. Dissociation Magnesium Hydroxide Formula.
From brainly.in
derive the formula of magnesium hydroxide using Criss cross method Dissociation Magnesium Hydroxide Formula When mg (oh)₂ dissolves in. Which chemical equation shows the dissociation of magnesium hydroxide? It consists of mg²⁺ ions and oh⁻ ions. Magnesium hydroxide is an ionic compound. (a) adding a common ion, mg 2+, will increase the concentration of this ion and shift the solubility equilibrium to the left, decreasing the. Magnesium hydroxide is an ionic compound, so when. Dissociation Magnesium Hydroxide Formula.
From www.coursehero.com
[Solved] Write the dissociation equation for the dissolving of Dissociation Magnesium Hydroxide Formula In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. It consists of mg²⁺ ions and oh⁻ ions. Its formula is mg (oh)₂. Thus, magnesium hydroxide is an electrolyte. Magnesium hydroxide is an ionic compound. Which chemical equation shows the dissociation of magnesium hydroxide? (a) adding a common ion, mg. Dissociation Magnesium Hydroxide Formula.
From www.chegg.com
Solved QUESTION 1See PQ14 1. Dissociation of magnesium Dissociation Magnesium Hydroxide Formula Which chemical equation shows the dissociation of magnesium hydroxide? In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. When mg (oh)₂ dissolves in. Magnesium hydroxide is an ionic compound, so when it dissolves it dissociates. Magnesium hydroxide is an ionic compound. Thus, magnesium hydroxide is an electrolyte. Its formula. Dissociation Magnesium Hydroxide Formula.
From www.slideserve.com
PPT Mg(OH) 2 + 2HCl → 2H 2 O + MgCl 2 PowerPoint Presentation, free Dissociation Magnesium Hydroxide Formula (a) adding a common ion, mg 2+, will increase the concentration of this ion and shift the solubility equilibrium to the left, decreasing the. It consists of mg²⁺ ions and oh⁻ ions. Magnesium hydroxide is an ionic compound, so when it dissolves it dissociates. Thus, magnesium hydroxide is an electrolyte. When mg (oh)₂ dissolves in. In this video we will. Dissociation Magnesium Hydroxide Formula.
From www.youtube.com
Equation for Mg(NO3)2 + H2O (Magnesium nitrate + Water) YouTube Dissociation Magnesium Hydroxide Formula Its formula is mg (oh)₂. Magnesium hydroxide is an ionic compound, so when it dissolves it dissociates. Which chemical equation shows the dissociation of magnesium hydroxide? (a) adding a common ion, mg 2+, will increase the concentration of this ion and shift the solubility equilibrium to the left, decreasing the. Thus, magnesium hydroxide is an electrolyte. Magnesium hydroxide is an. Dissociation Magnesium Hydroxide Formula.
From www.numerade.com
SOLVED write a balanced chemical equation for the dissociation of Dissociation Magnesium Hydroxide Formula Magnesium hydroxide is an ionic compound, so when it dissolves it dissociates. When mg (oh)₂ dissolves in. Magnesium hydroxide is an ionic compound. Thus, magnesium hydroxide is an electrolyte. In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. Which chemical equation shows the dissociation of magnesium hydroxide? Its formula. Dissociation Magnesium Hydroxide Formula.
From www.shutterstock.com
Magnesium Hydroxide Formula Handwritten Chemical Formula Stock Dissociation Magnesium Hydroxide Formula Thus, magnesium hydroxide is an electrolyte. Which chemical equation shows the dissociation of magnesium hydroxide? When mg (oh)₂ dissolves in. It consists of mg²⁺ ions and oh⁻ ions. In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. Magnesium hydroxide is an ionic compound, so when it dissolves it dissociates.. Dissociation Magnesium Hydroxide Formula.
From www.youtube.com
How to Write the Formula for Magnesium hydroxide YouTube Dissociation Magnesium Hydroxide Formula (a) adding a common ion, mg 2+, will increase the concentration of this ion and shift the solubility equilibrium to the left, decreasing the. It consists of mg²⁺ ions and oh⁻ ions. Which chemical equation shows the dissociation of magnesium hydroxide? Its formula is mg (oh)₂. When mg (oh)₂ dissolves in. Magnesium hydroxide is an ionic compound. Thus, magnesium hydroxide. Dissociation Magnesium Hydroxide Formula.
From animalia-life.club
Magnesium Hydroxide Formula Dissociation Magnesium Hydroxide Formula (a) adding a common ion, mg 2+, will increase the concentration of this ion and shift the solubility equilibrium to the left, decreasing the. Magnesium hydroxide is an ionic compound. In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. Thus, magnesium hydroxide is an electrolyte. It consists of mg²⁺. Dissociation Magnesium Hydroxide Formula.
From www.youtube.com
Equation for Magnesium Hydroxide Dissolving in Water Mg(OH)2 + H2O Dissociation Magnesium Hydroxide Formula Thus, magnesium hydroxide is an electrolyte. (a) adding a common ion, mg 2+, will increase the concentration of this ion and shift the solubility equilibrium to the left, decreasing the. When mg (oh)₂ dissolves in. In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. Magnesium hydroxide is an ionic. Dissociation Magnesium Hydroxide Formula.
From www.bartleby.com
Answered The equation for the dissolving of… bartleby Dissociation Magnesium Hydroxide Formula Which chemical equation shows the dissociation of magnesium hydroxide? (a) adding a common ion, mg 2+, will increase the concentration of this ion and shift the solubility equilibrium to the left, decreasing the. Magnesium hydroxide is an ionic compound, so when it dissolves it dissociates. When mg (oh)₂ dissolves in. Its formula is mg (oh)₂. Magnesium hydroxide is an ionic. Dissociation Magnesium Hydroxide Formula.
From loejapewb.blob.core.windows.net
Magnesium Hydroxide at Diane Kruse blog Dissociation Magnesium Hydroxide Formula In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. It consists of mg²⁺ ions and oh⁻ ions. Magnesium hydroxide is an ionic compound, so when it dissolves it dissociates. Its formula is mg (oh)₂. Magnesium hydroxide is an ionic compound. Which chemical equation shows the dissociation of magnesium hydroxide?. Dissociation Magnesium Hydroxide Formula.
From www.shutterstock.com
Magnesium Hydroxide Formula Handwritten Chemical Formula Stock Dissociation Magnesium Hydroxide Formula Thus, magnesium hydroxide is an electrolyte. Magnesium hydroxide is an ionic compound. Magnesium hydroxide is an ionic compound, so when it dissolves it dissociates. It consists of mg²⁺ ions and oh⁻ ions. Which chemical equation shows the dissociation of magnesium hydroxide? When mg (oh)₂ dissolves in. In this video we will describe the equation mg (oh)2 + h2o and write. Dissociation Magnesium Hydroxide Formula.
From www.slideserve.com
PPT Chapter 5 Molecular View of Reactions in Aqueous Solutions Part I Dissociation Magnesium Hydroxide Formula In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. It consists of mg²⁺ ions and oh⁻ ions. Thus, magnesium hydroxide is an electrolyte. (a) adding a common ion, mg 2+, will increase the concentration of this ion and shift the solubility equilibrium to the left, decreasing the. Magnesium hydroxide. Dissociation Magnesium Hydroxide Formula.
From www.youtube.com
Biochemistry 3.2 Dissociation of acids YouTube Dissociation Magnesium Hydroxide Formula Its formula is mg (oh)₂. Magnesium hydroxide is an ionic compound, so when it dissolves it dissociates. Magnesium hydroxide is an ionic compound. (a) adding a common ion, mg 2+, will increase the concentration of this ion and shift the solubility equilibrium to the left, decreasing the. Thus, magnesium hydroxide is an electrolyte. When mg (oh)₂ dissolves in. In this. Dissociation Magnesium Hydroxide Formula.
From childhealthpolicy.vumc.org
⛔ Chemical reaction of magnesium and oxygen. What is the chemical Dissociation Magnesium Hydroxide Formula When mg (oh)₂ dissolves in. Magnesium hydroxide is an ionic compound. Its formula is mg (oh)₂. It consists of mg²⁺ ions and oh⁻ ions. Which chemical equation shows the dissociation of magnesium hydroxide? In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. (a) adding a common ion, mg 2+,. Dissociation Magnesium Hydroxide Formula.
From infinitylearn.com
Magnesium hydroxide Formula Infinity Learn Dissociation Magnesium Hydroxide Formula It consists of mg²⁺ ions and oh⁻ ions. In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. Thus, magnesium hydroxide is an electrolyte. Magnesium hydroxide is an ionic compound, so when it dissolves it dissociates. Which chemical equation shows the dissociation of magnesium hydroxide? Magnesium hydroxide is an ionic. Dissociation Magnesium Hydroxide Formula.
From www.numerade.com
SOLVED Write net ionic equation for the reaction that occurs when Dissociation Magnesium Hydroxide Formula Which chemical equation shows the dissociation of magnesium hydroxide? It consists of mg²⁺ ions and oh⁻ ions. Thus, magnesium hydroxide is an electrolyte. In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. Its formula is mg (oh)₂. (a) adding a common ion, mg 2+, will increase the concentration of. Dissociation Magnesium Hydroxide Formula.
From ceyfadjf.blob.core.windows.net
Magnesium Hydroxide Soluble Formula at Edwin Mcbride blog Dissociation Magnesium Hydroxide Formula Which chemical equation shows the dissociation of magnesium hydroxide? Thus, magnesium hydroxide is an electrolyte. Magnesium hydroxide is an ionic compound, so when it dissolves it dissociates. Its formula is mg (oh)₂. Magnesium hydroxide is an ionic compound. (a) adding a common ion, mg 2+, will increase the concentration of this ion and shift the solubility equilibrium to the left,. Dissociation Magnesium Hydroxide Formula.
From www.dreamstime.com
Magnesium Hydroxide Molecule 3d Rendering, Flat Molecular Structure Dissociation Magnesium Hydroxide Formula (a) adding a common ion, mg 2+, will increase the concentration of this ion and shift the solubility equilibrium to the left, decreasing the. Magnesium hydroxide is an ionic compound. When mg (oh)₂ dissolves in. In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. Magnesium hydroxide is an ionic. Dissociation Magnesium Hydroxide Formula.
From www.numerade.com
SOLVED Write chemical equation for the dissociation of each of the Dissociation Magnesium Hydroxide Formula It consists of mg²⁺ ions and oh⁻ ions. Thus, magnesium hydroxide is an electrolyte. When mg (oh)₂ dissolves in. Its formula is mg (oh)₂. Magnesium hydroxide is an ionic compound, so when it dissolves it dissociates. Magnesium hydroxide is an ionic compound. (a) adding a common ion, mg 2+, will increase the concentration of this ion and shift the solubility. Dissociation Magnesium Hydroxide Formula.
From lilynewsbranch.blogspot.com
Which Chemical Equation Shows the Dissociation of Magnesium Hydroxide Dissociation Magnesium Hydroxide Formula Magnesium hydroxide is an ionic compound, so when it dissolves it dissociates. In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. Thus, magnesium hydroxide is an electrolyte. When mg (oh)₂ dissolves in. It consists of mg²⁺ ions and oh⁻ ions. (a) adding a common ion, mg 2+, will increase. Dissociation Magnesium Hydroxide Formula.