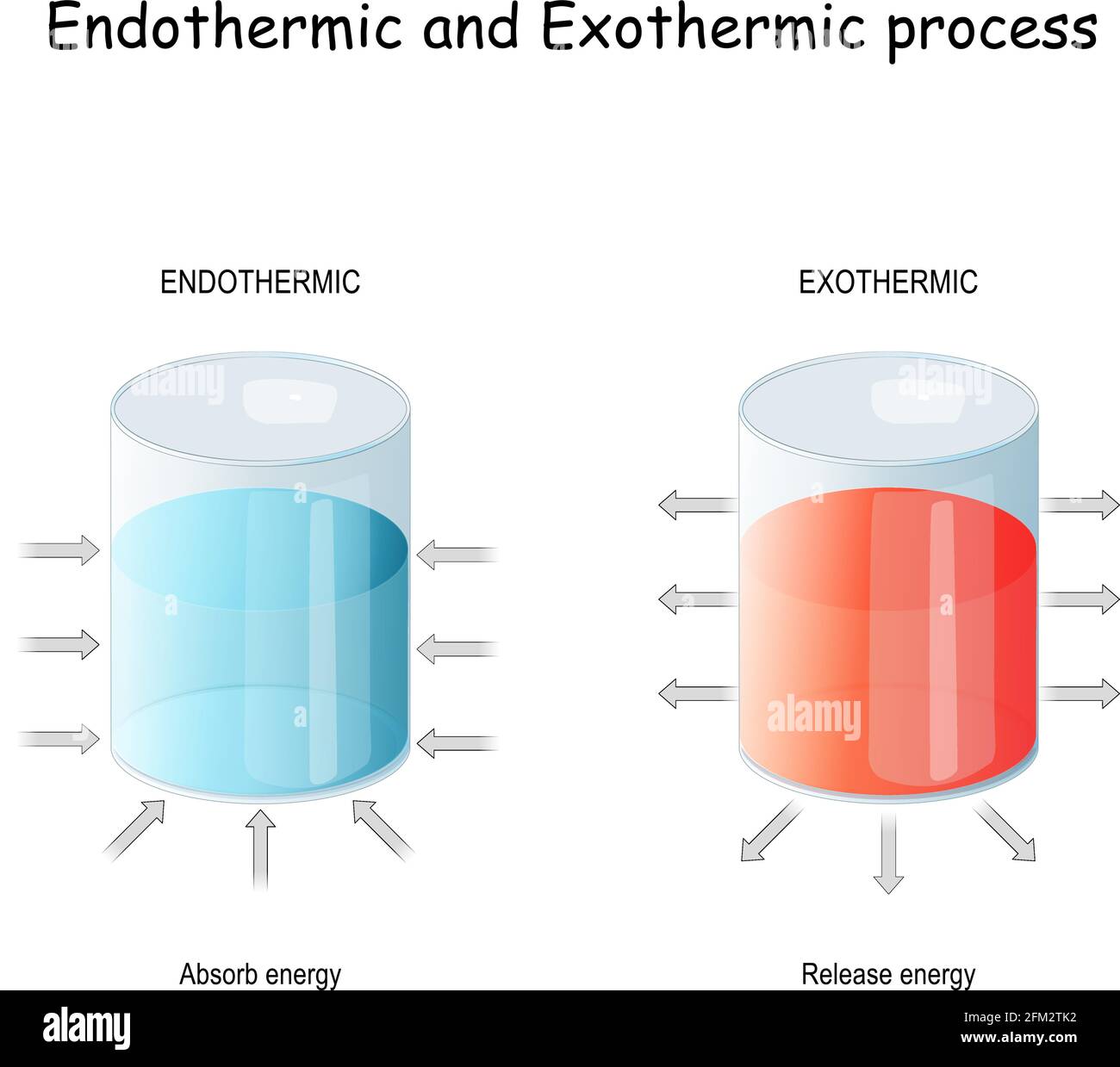
Endothermic Reaction Are . In endothermic reactions, more energy is absorbed when the. A hallmark of this type of reaction is that it feels cold. \text{mol}\) of calcium carbonate decomposes into \(1 \: \text{mol}\) of calcium oxide and \(1 \:. In endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a product. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. An endothermic reaction is any chemical reaction that absorbs heat from its environment. Photosynthesis is a good example of an endothermic. An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. The absorbed energy provides the activation energy for the reaction to occur. Chemical reactions that absorb (or use) energy overall are called endothermic.
from www.animalia-life.club
An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. A hallmark of this type of reaction is that it feels cold. In endothermic reactions, more energy is absorbed when the. Photosynthesis is a good example of an endothermic. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. In endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a product. \text{mol}\) of calcium carbonate decomposes into \(1 \: The absorbed energy provides the activation energy for the reaction to occur. An endothermic reaction is any chemical reaction that absorbs heat from its environment. \text{mol}\) of calcium oxide and \(1 \:.
Endothermic Reaction Examples For Kids
Endothermic Reaction Are The absorbed energy provides the activation energy for the reaction to occur. \text{mol}\) of calcium carbonate decomposes into \(1 \: A hallmark of this type of reaction is that it feels cold. An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Photosynthesis is a good example of an endothermic. In endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a product. An endothermic reaction is any chemical reaction that absorbs heat from its environment. The absorbed energy provides the activation energy for the reaction to occur. \text{mol}\) of calcium oxide and \(1 \:. In endothermic reactions, more energy is absorbed when the. Chemical reactions that absorb (or use) energy overall are called endothermic.
From www.youtube.com
Endothermic & Exothermic reaction class 10 chemical reaction and Endothermic Reaction Are \text{mol}\) of calcium carbonate decomposes into \(1 \: An endothermic reaction is any chemical reaction that absorbs heat from its environment. \text{mol}\) of calcium oxide and \(1 \:. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. In. Endothermic Reaction Are.
From loehrbtpf.blob.core.windows.net
Endothermic Reaction Thermal at John Maxey blog Endothermic Reaction Are Chemical reactions that absorb (or use) energy overall are called endothermic. In endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a product. An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. In endothermic reactions, more energy is absorbed when the. The absorbed. Endothermic Reaction Are.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Are The absorbed energy provides the activation energy for the reaction to occur. An endothermic reaction is any chemical reaction that absorbs heat from its environment. Chemical reactions that absorb (or use) energy overall are called endothermic. \text{mol}\) of calcium oxide and \(1 \:. A hallmark of this type of reaction is that it feels cold. Endothermic and exothermic reactions are. Endothermic Reaction Are.
From byjus.com
24. What is the Minimum activation energy required for endothermic and Endothermic Reaction Are Chemical reactions that absorb (or use) energy overall are called endothermic. In endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a product. In endothermic reactions, more energy is absorbed when the. \text{mol}\) of calcium carbonate decomposes into \(1 \: An endothermic reaction is any chemical reaction that absorbs heat from its. Endothermic Reaction Are.
From www.slideserve.com
PPT Exothermic and endothermic reactions PowerPoint Presentation Endothermic Reaction Are A hallmark of this type of reaction is that it feels cold. An endothermic reaction is any chemical reaction that absorbs heat from its environment. Chemical reactions that absorb (or use) energy overall are called endothermic. Photosynthesis is a good example of an endothermic. \text{mol}\) of calcium carbonate decomposes into \(1 \: An endothermic reaction is one in which the. Endothermic Reaction Are.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction Are Chemical reactions that absorb (or use) energy overall are called endothermic. Photosynthesis is a good example of an endothermic. An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. \text{mol}\) of calcium carbonate decomposes into \(1 \: The absorbed energy provides the activation energy for the reaction to occur. In endothermic reactions,. Endothermic Reaction Are.
From gamesmartz.com
Endothermic Reaction Easy to Understand Endothermic Reaction Are An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. The absorbed energy provides the activation energy for the reaction to occur. An endothermic reaction is any chemical reaction that absorbs heat from its environment. In endothermic reactions, more. Endothermic Reaction Are.
From www.studyorgo.com
How to Interpret Thermodynamics of Reactions Endothermic Reaction Are In endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a product. An endothermic reaction is any chemical reaction that absorbs heat from its environment. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Photosynthesis is a good example of an endothermic. \text{mol}\) of calcium carbonate decomposes into. Endothermic Reaction Are.
From klamequqc.blob.core.windows.net
Endothermic Reaction Used In A Sentence at Terrence Rankin blog Endothermic Reaction Are Chemical reactions that absorb (or use) energy overall are called endothermic. \text{mol}\) of calcium oxide and \(1 \:. An endothermic reaction is any chemical reaction that absorbs heat from its environment. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Photosynthesis is a good example of an endothermic. In endothermic reactions, more energy is absorbed when. Endothermic Reaction Are.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Reaction Are Photosynthesis is a good example of an endothermic. Chemical reactions that absorb (or use) energy overall are called endothermic. An endothermic reaction is any chemical reaction that absorbs heat from its environment. In endothermic reactions, more energy is absorbed when the. \text{mol}\) of calcium carbonate decomposes into \(1 \: The absorbed energy provides the activation energy for the reaction to. Endothermic Reaction Are.
From pediaa.com
Difference Between Endothermic and Exothermic Reactions Definition Endothermic Reaction Are Chemical reactions that absorb (or use) energy overall are called endothermic. An endothermic reaction is any chemical reaction that absorbs heat from its environment. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. A hallmark of this type of reaction is that it feels cold. The absorbed energy provides the activation energy for the reaction to. Endothermic Reaction Are.
From resolutionsforyou.com
The Journey of an Endothermic Reaction Understanding the Reaction Endothermic Reaction Are In endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a product. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. A hallmark of this type of reaction is that it feels cold. An endothermic reaction is any chemical reaction that absorbs heat from its environment. \text{mol}\) of. Endothermic Reaction Are.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Endothermic Reaction Are \text{mol}\) of calcium oxide and \(1 \:. \text{mol}\) of calcium carbonate decomposes into \(1 \: Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. A hallmark of this type of reaction is that it feels cold. Chemical reactions. Endothermic Reaction Are.
From slideplayer.com
Endothermic and exothermic reactions ppt download Endothermic Reaction Are In endothermic reactions, more energy is absorbed when the. In endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a product. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases.. Endothermic Reaction Are.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Are An endothermic reaction is any chemical reaction that absorbs heat from its environment. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. The absorbed energy provides the activation energy for the reaction to occur. \text{mol}\) of calcium oxide and \(1 \:. A hallmark of this type of reaction is that it feels cold. Photosynthesis is a. Endothermic Reaction Are.
From mmerevise.co.uk
Endothermic and Exothermic Reactions Revision MME Endothermic Reaction Are A hallmark of this type of reaction is that it feels cold. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Chemical reactions that absorb (or use) energy overall are called endothermic. Photosynthesis is a good example of an endothermic. In endothermic and exothermic reactions, energy can be thought of as either a reactant of the. Endothermic Reaction Are.
From khatiar1955a.altervista.org
The Difference Between Endothermic and Exothermic Reactions Endothermic Reaction Are \text{mol}\) of calcium carbonate decomposes into \(1 \: Chemical reactions that absorb (or use) energy overall are called endothermic. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. The absorbed energy provides the activation energy for the reaction to occur. \text{mol}\) of calcium oxide and \(1 \:. Photosynthesis is a good example of an endothermic. In. Endothermic Reaction Are.
From www.thoughtco.com
Endothermic and Exothermic Chemical Reactions Endothermic Reaction Are An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. In endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a product. \text{mol}\) of calcium carbonate decomposes into \(1 \: In endothermic reactions, more energy is absorbed when the. The absorbed energy provides the. Endothermic Reaction Are.
From question.pandai.org
Endothermic and exothermic reactions Endothermic Reaction Are Chemical reactions that absorb (or use) energy overall are called endothermic. Photosynthesis is a good example of an endothermic. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. An endothermic reaction is any chemical reaction that absorbs heat from its environment. The absorbed energy provides the activation energy for the reaction to occur. In endothermic and. Endothermic Reaction Are.
From www.thoughtco.com
Endothermic Reaction Examples Endothermic Reaction Are A hallmark of this type of reaction is that it feels cold. \text{mol}\) of calcium carbonate decomposes into \(1 \: Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. \text{mol}\) of calcium oxide and \(1 \:. Chemical reactions that absorb (or use) energy overall are called endothermic. An endothermic reaction is one in which the enthalpy. Endothermic Reaction Are.
From www.animalia-life.club
Endothermic Reaction Examples For Kids Endothermic Reaction Are An endothermic reaction is any chemical reaction that absorbs heat from its environment. The absorbed energy provides the activation energy for the reaction to occur. Chemical reactions that absorb (or use) energy overall are called endothermic. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Photosynthesis is a good example of an endothermic. In endothermic reactions,. Endothermic Reaction Are.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Reaction Are Photosynthesis is a good example of an endothermic. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. \text{mol}\) of calcium carbonate decomposes into \(1 \: In endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a product. In endothermic reactions, more energy is absorbed when the. An endothermic. Endothermic Reaction Are.
From exywpuoix.blob.core.windows.net
Ice Pack Endothermic Or Exothermic Reaction at Vivian Dougherty blog Endothermic Reaction Are The absorbed energy provides the activation energy for the reaction to occur. Photosynthesis is a good example of an endothermic. In endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a product. \text{mol}\) of calcium carbonate decomposes into \(1 \: In endothermic reactions, more energy is absorbed when the. An endothermic reaction. Endothermic Reaction Are.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endothermic Reaction Are Chemical reactions that absorb (or use) energy overall are called endothermic. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. In endothermic reactions, more energy is absorbed when the. An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. An endothermic reaction is any chemical reaction that absorbs. Endothermic Reaction Are.
From www.slideserve.com
PPT Endothermic Vs. Exothermic Reaction Graphs PowerPoint Endothermic Reaction Are Chemical reactions that absorb (or use) energy overall are called endothermic. A hallmark of this type of reaction is that it feels cold. An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. Photosynthesis is a good example of an endothermic. In endothermic reactions, more energy is absorbed when the. In endothermic. Endothermic Reaction Are.
From www.slideserve.com
PPT Endothermic and Exothermic Reactions PowerPoint Presentation Endothermic Reaction Are In endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a product. \text{mol}\) of calcium carbonate decomposes into \(1 \: Photosynthesis is a good example of an endothermic. In endothermic reactions, more energy is absorbed when the. An endothermic reaction is any chemical reaction that absorbs heat from its environment. Endothermic and. Endothermic Reaction Are.
From www.scribd.com
Endothermic Reaction Exothermic Reaction Delta H Delta H PDF Endothermic Reaction Are An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. \text{mol}\) of calcium oxide and \(1 \:. An endothermic reaction is any chemical reaction that absorbs heat from its environment. Chemical reactions that absorb (or use) energy overall are called endothermic. A hallmark of this type of reaction is that it feels. Endothermic Reaction Are.
From www.slideserve.com
PPT Endothermic Vs. Exothermic Reaction Graphs PowerPoint Endothermic Reaction Are \text{mol}\) of calcium carbonate decomposes into \(1 \: In endothermic reactions, more energy is absorbed when the. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. An endothermic reaction is any chemical reaction that absorbs heat from its environment. In endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction. Endothermic Reaction Are.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reaction Are An endothermic reaction is any chemical reaction that absorbs heat from its environment. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. In endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a product. In endothermic reactions, more energy is absorbed when the. Chemical reactions that absorb (or. Endothermic Reaction Are.
From mavink.com
Draw An Energy Level Diagram For An Endothermic Reaction Endothermic Reaction Are An endothermic reaction is any chemical reaction that absorbs heat from its environment. In endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a product. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. \text{mol}\) of calcium oxide and \(1 \:. \text{mol}\) of calcium carbonate decomposes into \(1. Endothermic Reaction Are.
From www.youtube.com
Endothermic Reactions YouTube Endothermic Reaction Are Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. \text{mol}\) of calcium oxide and \(1 \:. A hallmark of this type of reaction is that it feels cold. The absorbed energy provides the activation energy for the reaction to occur. In endothermic and exothermic reactions, energy can be thought of as either a reactant of the. Endothermic Reaction Are.
From loehrbtpf.blob.core.windows.net
Endothermic Reaction Thermal at John Maxey blog Endothermic Reaction Are In endothermic reactions, more energy is absorbed when the. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. \text{mol}\) of calcium carbonate decomposes into \(1 \: Chemical reactions that absorb (or use) energy overall are called endothermic. An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. An. Endothermic Reaction Are.
From h-o-m-e.org
Chilling with Endothermic Reactions Endothermic Reaction Are The absorbed energy provides the activation energy for the reaction to occur. An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. An endothermic reaction is any chemical reaction that absorbs heat from its environment. Photosynthesis is a good example of an endothermic. Endothermic and exothermic reactions are chemical reactions that absorb. Endothermic Reaction Are.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Are In endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a product. An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. The absorbed energy provides the activation energy for the reaction to occur. Chemical reactions that absorb (or use) energy overall are called. Endothermic Reaction Are.
From www.slideserve.com
PPT Exo and Endothermic Reactions PowerPoint Presentation, free Endothermic Reaction Are Chemical reactions that absorb (or use) energy overall are called endothermic. Photosynthesis is a good example of an endothermic. In endothermic reactions, more energy is absorbed when the. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. In endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. Endothermic Reaction Are.