What Is Ecell In Chemistry . The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. When both reactants and products are in their standard states, the relationship between δg° and \(e^°_{cell}\) is as follows:. E° cell is the electromotive force (also called cell voltage or cell potential) between two half. The ecell formula tells you how to. The potential difference is caused by the ability of. Cell potential refers to the voltage of an electrochemical cell whose value can be affected by temperature, concentration, and pressure. Interpret electrode potentials in terms of relative oxidant and reductant. The standard cell potential is the value. Calculating the potential of an e cell means figuring out which reactions are going to occur. Describe and relate the definitions of electrode and cell potentials.
from slidetodoc.com
Describe and relate the definitions of electrode and cell potentials. Calculating the potential of an e cell means figuring out which reactions are going to occur. When both reactants and products are in their standard states, the relationship between δg° and \(e^°_{cell}\) is as follows:. The standard cell potential is the value. Interpret electrode potentials in terms of relative oxidant and reductant. Cell potential refers to the voltage of an electrochemical cell whose value can be affected by temperature, concentration, and pressure. The potential difference is caused by the ability of. E° cell is the electromotive force (also called cell voltage or cell potential) between two half. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The ecell formula tells you how to.
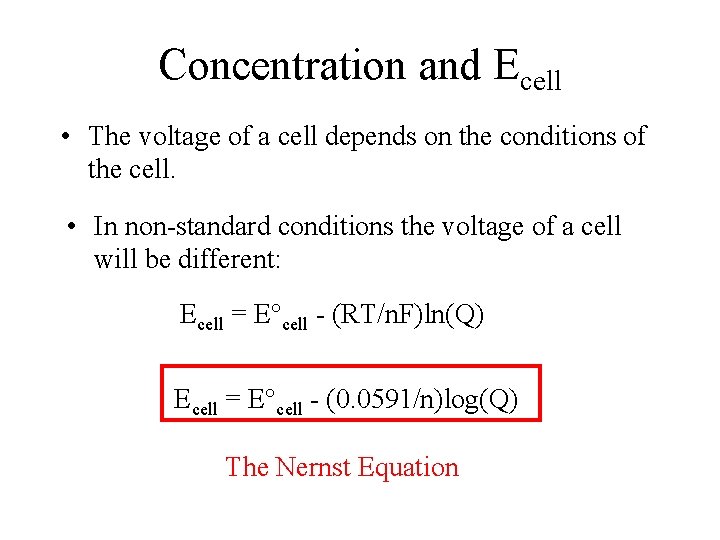
The Nernst Equation Concentration and Ecell The voltage
What Is Ecell In Chemistry E° cell is the electromotive force (also called cell voltage or cell potential) between two half. Describe and relate the definitions of electrode and cell potentials. E° cell is the electromotive force (also called cell voltage or cell potential) between two half. The potential difference is caused by the ability of. Interpret electrode potentials in terms of relative oxidant and reductant. Cell potential refers to the voltage of an electrochemical cell whose value can be affected by temperature, concentration, and pressure. When both reactants and products are in their standard states, the relationship between δg° and \(e^°_{cell}\) is as follows:. The standard cell potential is the value. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The ecell formula tells you how to. Calculating the potential of an e cell means figuring out which reactions are going to occur.
From scienceinfo.com
Cell Potential & Standard Cell Potential What Is Ecell In Chemistry The ecell formula tells you how to. Cell potential refers to the voltage of an electrochemical cell whose value can be affected by temperature, concentration, and pressure. Calculating the potential of an e cell means figuring out which reactions are going to occur. Interpret electrode potentials in terms of relative oxidant and reductant. The cell potential, \(e_{cell}\), is the measure. What Is Ecell In Chemistry.
From educationnerved.z4.web.core.windows.net
How To Calculate E Cell Chemistry What Is Ecell In Chemistry E° cell is the electromotive force (also called cell voltage or cell potential) between two half. Cell potential refers to the voltage of an electrochemical cell whose value can be affected by temperature, concentration, and pressure. The ecell formula tells you how to. Calculating the potential of an e cell means figuring out which reactions are going to occur. Interpret. What Is Ecell In Chemistry.
From www.showme.com
Ecell, Delta G, and the equilibrium constant Science, AP Chemistry What Is Ecell In Chemistry Interpret electrode potentials in terms of relative oxidant and reductant. Cell potential refers to the voltage of an electrochemical cell whose value can be affected by temperature, concentration, and pressure. Calculating the potential of an e cell means figuring out which reactions are going to occur. The ecell formula tells you how to. The potential difference is caused by the. What Is Ecell In Chemistry.
From www.careers360.com
Electrochemical Cell Definition, Examples, Types, Uses, FAQs What Is Ecell In Chemistry The ecell formula tells you how to. E° cell is the electromotive force (also called cell voltage or cell potential) between two half. The standard cell potential is the value. Describe and relate the definitions of electrode and cell potentials. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Cell. What Is Ecell In Chemistry.
From www.chegg.com
Solved Using the Standard Cell Potential Equation, Ecell = What Is Ecell In Chemistry When both reactants and products are in their standard states, the relationship between δg° and \(e^°_{cell}\) is as follows:. Interpret electrode potentials in terms of relative oxidant and reductant. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Describe and relate the definitions of electrode and cell potentials. E° cell. What Is Ecell In Chemistry.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free What Is Ecell In Chemistry Interpret electrode potentials in terms of relative oxidant and reductant. When both reactants and products are in their standard states, the relationship between δg° and \(e^°_{cell}\) is as follows:. The ecell formula tells you how to. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. E° cell is the electromotive. What Is Ecell In Chemistry.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells What Is Ecell In Chemistry When both reactants and products are in their standard states, the relationship between δg° and \(e^°_{cell}\) is as follows:. The ecell formula tells you how to. The potential difference is caused by the ability of. Calculating the potential of an e cell means figuring out which reactions are going to occur. Cell potential refers to the voltage of an electrochemical. What Is Ecell In Chemistry.
From www.youtube.com
Calculating Ecell YouTube What Is Ecell In Chemistry The potential difference is caused by the ability of. Cell potential refers to the voltage of an electrochemical cell whose value can be affected by temperature, concentration, and pressure. Calculating the potential of an e cell means figuring out which reactions are going to occur. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells. What Is Ecell In Chemistry.
From www.showme.com
Calculating Ecell Chemistry, Science ShowMe What Is Ecell In Chemistry Cell potential refers to the voltage of an electrochemical cell whose value can be affected by temperature, concentration, and pressure. Interpret electrode potentials in terms of relative oxidant and reductant. The potential difference is caused by the ability of. Calculating the potential of an e cell means figuring out which reactions are going to occur. When both reactants and products. What Is Ecell In Chemistry.
From studymarxianism.z21.web.core.windows.net
How To Calculate E Cell Chemistry What Is Ecell In Chemistry The ecell formula tells you how to. The potential difference is caused by the ability of. Cell potential refers to the voltage of an electrochemical cell whose value can be affected by temperature, concentration, and pressure. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Describe and relate the definitions. What Is Ecell In Chemistry.
From educationnerved.z4.web.core.windows.net
How To Calculate E Cell Chemistry What Is Ecell In Chemistry The ecell formula tells you how to. Describe and relate the definitions of electrode and cell potentials. Cell potential refers to the voltage of an electrochemical cell whose value can be affected by temperature, concentration, and pressure. Calculating the potential of an e cell means figuring out which reactions are going to occur. The cell potential, \(e_{cell}\), is the measure. What Is Ecell In Chemistry.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types What Is Ecell In Chemistry Interpret electrode potentials in terms of relative oxidant and reductant. The potential difference is caused by the ability of. E° cell is the electromotive force (also called cell voltage or cell potential) between two half. When both reactants and products are in their standard states, the relationship between δg° and \(e^°_{cell}\) is as follows:. Calculating the potential of an e. What Is Ecell In Chemistry.
From www.youtube.com
Electrochemical Cell Notation YouTube What Is Ecell In Chemistry E° cell is the electromotive force (also called cell voltage or cell potential) between two half. Calculating the potential of an e cell means figuring out which reactions are going to occur. The ecell formula tells you how to. Describe and relate the definitions of electrode and cell potentials. The standard cell potential is the value. The potential difference is. What Is Ecell In Chemistry.
From studylib.net
Electrochemical cells What Is Ecell In Chemistry The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. When both reactants and products are in their standard states, the relationship between δg° and \(e^°_{cell}\) is as follows:. Describe and relate the definitions of electrode and cell potentials. The potential difference is caused by the ability of. E° cell is. What Is Ecell In Chemistry.
From wisc.pb.unizin.org
D39.4 Introduction to Voltaic Cells Chemistry 109 Fall 2021 What Is Ecell In Chemistry Cell potential refers to the voltage of an electrochemical cell whose value can be affected by temperature, concentration, and pressure. Calculating the potential of an e cell means figuring out which reactions are going to occur. E° cell is the electromotive force (also called cell voltage or cell potential) between two half. The ecell formula tells you how to. The. What Is Ecell In Chemistry.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells What Is Ecell In Chemistry The standard cell potential is the value. The ecell formula tells you how to. Cell potential refers to the voltage of an electrochemical cell whose value can be affected by temperature, concentration, and pressure. Interpret electrode potentials in terms of relative oxidant and reductant. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in. What Is Ecell In Chemistry.
From www.showme.com
Calculating Ecell value Science, AP Chemistry ShowMe What Is Ecell In Chemistry Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. The ecell formula tells you how to. When both reactants and products are in their standard states, the relationship between δg° and \(e^°_{cell}\) is as follows:. E° cell is the electromotive force (also called cell voltage or cell potential) between. What Is Ecell In Chemistry.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry for Majors What Is Ecell In Chemistry Interpret electrode potentials in terms of relative oxidant and reductant. When both reactants and products are in their standard states, the relationship between δg° and \(e^°_{cell}\) is as follows:. Describe and relate the definitions of electrode and cell potentials. Cell potential refers to the voltage of an electrochemical cell whose value can be affected by temperature, concentration, and pressure. The. What Is Ecell In Chemistry.
From www.pinterest.com
Electrochemical Cells Chemwiki Chemistry Textbook, Gcse Chemistry What Is Ecell In Chemistry Cell potential refers to the voltage of an electrochemical cell whose value can be affected by temperature, concentration, and pressure. The standard cell potential is the value. E° cell is the electromotive force (also called cell voltage or cell potential) between two half. Describe and relate the definitions of electrode and cell potentials. When both reactants and products are in. What Is Ecell In Chemistry.
From www.pinterest.com
Difference Between Electrochemical Cell and Electrolytic Cell What Is Ecell In Chemistry When both reactants and products are in their standard states, the relationship between δg° and \(e^°_{cell}\) is as follows:. The ecell formula tells you how to. Describe and relate the definitions of electrode and cell potentials. E° cell is the electromotive force (also called cell voltage or cell potential) between two half. Calculating the potential of an e cell means. What Is Ecell In Chemistry.
From www.youtube.com
Emf of daniel cell from nernst equation(Electrochemistry part 28 for What Is Ecell In Chemistry When both reactants and products are in their standard states, the relationship between δg° and \(e^°_{cell}\) is as follows:. The potential difference is caused by the ability of. The ecell formula tells you how to. Calculating the potential of an e cell means figuring out which reactions are going to occur. Describe and relate the definitions of electrode and cell. What Is Ecell In Chemistry.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General What Is Ecell In Chemistry Describe and relate the definitions of electrode and cell potentials. When both reactants and products are in their standard states, the relationship between δg° and \(e^°_{cell}\) is as follows:. E° cell is the electromotive force (also called cell voltage or cell potential) between two half. The standard cell potential is the value. Cell potential refers to the voltage of an. What Is Ecell In Chemistry.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts What Is Ecell In Chemistry The ecell formula tells you how to. Describe and relate the definitions of electrode and cell potentials. The potential difference is caused by the ability of. The standard cell potential is the value. Calculating the potential of an e cell means figuring out which reactions are going to occur. E° cell is the electromotive force (also called cell voltage or. What Is Ecell In Chemistry.
From quizspattering.z21.web.core.windows.net
How To Calculate E Cell Chemistry What Is Ecell In Chemistry The ecell formula tells you how to. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Describe and relate the definitions of electrode and cell potentials. The potential difference is caused by the ability of. The standard cell potential is the value. When both reactants and products are in their. What Is Ecell In Chemistry.
From byjus.com
Define Ecell and E^°cell . and basic differences between them What Is Ecell In Chemistry The standard cell potential is the value. Interpret electrode potentials in terms of relative oxidant and reductant. When both reactants and products are in their standard states, the relationship between δg° and \(e^°_{cell}\) is as follows:. Calculating the potential of an e cell means figuring out which reactions are going to occur. E° cell is the electromotive force (also called. What Is Ecell In Chemistry.
From www.youtube.com
Worked example Calculating Ecell for the given cell What Is Ecell In Chemistry Describe and relate the definitions of electrode and cell potentials. E° cell is the electromotive force (also called cell voltage or cell potential) between two half. The standard cell potential is the value. Cell potential refers to the voltage of an electrochemical cell whose value can be affected by temperature, concentration, and pressure. Interpret electrode potentials in terms of relative. What Is Ecell In Chemistry.
From www.researchgate.net
Electrochemical reversible cell containing silver and zinc in What Is Ecell In Chemistry The potential difference is caused by the ability of. Interpret electrode potentials in terms of relative oxidant and reductant. The standard cell potential is the value. The ecell formula tells you how to. When both reactants and products are in their standard states, the relationship between δg° and \(e^°_{cell}\) is as follows:. Calculating the potential of an e cell means. What Is Ecell In Chemistry.
From in.pinterest.com
What is Electrochemical Cell Notation Line notation Cell Diagram What Is Ecell In Chemistry Interpret electrode potentials in terms of relative oxidant and reductant. The standard cell potential is the value. Calculating the potential of an e cell means figuring out which reactions are going to occur. The potential difference is caused by the ability of. Describe and relate the definitions of electrode and cell potentials. The cell potential, \(e_{cell}\), is the measure of. What Is Ecell In Chemistry.
From mavink.com
Diagram Of Electrochemical Cell What Is Ecell In Chemistry Cell potential refers to the voltage of an electrochemical cell whose value can be affected by temperature, concentration, and pressure. Interpret electrode potentials in terms of relative oxidant and reductant. E° cell is the electromotive force (also called cell voltage or cell potential) between two half. Calculating the potential of an e cell means figuring out which reactions are going. What Is Ecell In Chemistry.
From slidetodoc.com
The Nernst Equation Concentration and Ecell The voltage What Is Ecell In Chemistry Describe and relate the definitions of electrode and cell potentials. Cell potential refers to the voltage of an electrochemical cell whose value can be affected by temperature, concentration, and pressure. The ecell formula tells you how to. The potential difference is caused by the ability of. The standard cell potential is the value. The cell potential, \(e_{cell}\), is the measure. What Is Ecell In Chemistry.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID3975392 What Is Ecell In Chemistry Cell potential refers to the voltage of an electrochemical cell whose value can be affected by temperature, concentration, and pressure. Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. Calculating the potential of an e cell means figuring out which reactions are going to occur. E° cell is the. What Is Ecell In Chemistry.
From www.youtube.com
Nernst Equation Ecell Electrochemistry Physical Chemistry What Is Ecell In Chemistry The standard cell potential is the value. Describe and relate the definitions of electrode and cell potentials. Calculating the potential of an e cell means figuring out which reactions are going to occur. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Interpret electrode potentials in terms of relative oxidant. What Is Ecell In Chemistry.
From www.youtube.com
AP Chemistry Electrochemistry Cell Potentials YouTube What Is Ecell In Chemistry Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The ecell formula tells you how to. Calculating the potential of an e cell means figuring out which reactions are going. What Is Ecell In Chemistry.
From www.youtube.com
Calculate the Ecell of Al,Al+3,Ni+2,Ni part 16 electro chemistry What Is Ecell In Chemistry Interpret electrode potentials in terms of relative oxidant and reductant. The ecell formula tells you how to. Describe and relate the definitions of electrode and cell potentials. Calculating the potential of an e cell means figuring out which reactions are going to occur. Cell potential refers to the voltage of an electrochemical cell whose value can be affected by temperature,. What Is Ecell In Chemistry.
From www.youtube.com
Electrochemistry Ecell, G, and K YouTube What Is Ecell In Chemistry Describe and relate the definitions of electrode and cell potentials. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The potential difference is caused by the ability of. Calculating the potential of an e cell means figuring out which reactions are going to occur. The ecell formula tells you how. What Is Ecell In Chemistry.