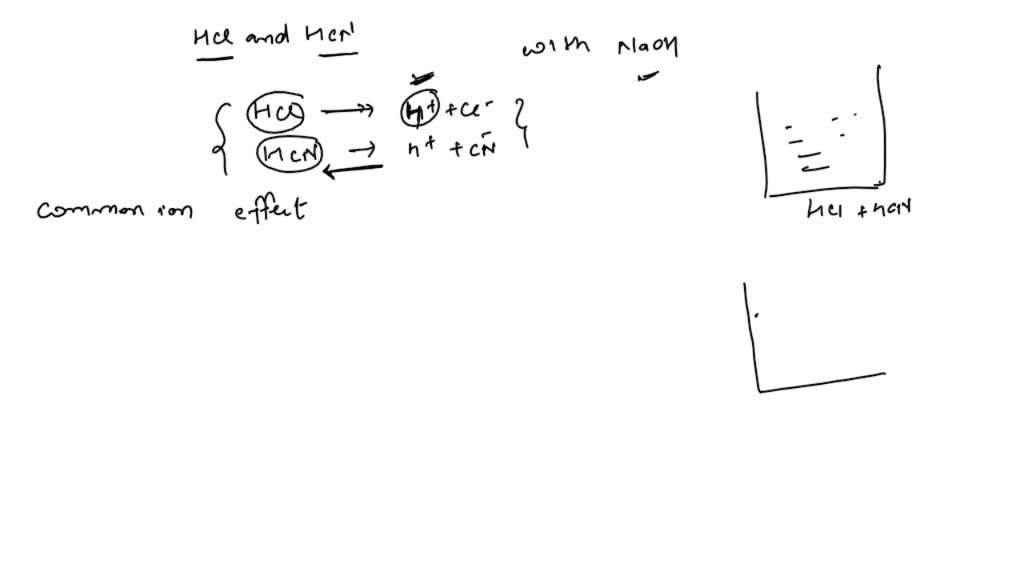
Conductometric Titration Of Hcl And Naoh . In this experiment you are going to perform a conductometric titration of a mixture of a strong acid and weak acid with a strong base. In first, the solution of hcl is unknown molarity; Strong acid with a strong base, e.g. Titration curve of a strong acid with a strong base, e.g. It is possible to find the concentration of. Before naoh is added, the conductance is. Before naoh is added, the conductance is high due to the presence of highly. 1) titration of strong acid (hcl) with strong base (naoh). Some typical conductometric titration curves are: Strong acid (hcl) and strong base (naoh) the observed shape of the titration curve (1) can be explained as follows:
from www.numerade.com
Before naoh is added, the conductance is high due to the presence of highly. Strong acid with a strong base, e.g. It is possible to find the concentration of. In first, the solution of hcl is unknown molarity; Before naoh is added, the conductance is. Titration curve of a strong acid with a strong base, e.g. Some typical conductometric titration curves are: In this experiment you are going to perform a conductometric titration of a mixture of a strong acid and weak acid with a strong base. 1) titration of strong acid (hcl) with strong base (naoh). Strong acid (hcl) and strong base (naoh) the observed shape of the titration curve (1) can be explained as follows:
SOLVEDConductometric titration curve of an equimolar mixture of a HCl
Conductometric Titration Of Hcl And Naoh Strong acid with a strong base, e.g. Some typical conductometric titration curves are: 1) titration of strong acid (hcl) with strong base (naoh). Before naoh is added, the conductance is high due to the presence of highly. It is possible to find the concentration of. In this experiment you are going to perform a conductometric titration of a mixture of a strong acid and weak acid with a strong base. In first, the solution of hcl is unknown molarity; Strong acid (hcl) and strong base (naoh) the observed shape of the titration curve (1) can be explained as follows: Titration curve of a strong acid with a strong base, e.g. Before naoh is added, the conductance is. Strong acid with a strong base, e.g.
From www.studypool.com
SOLUTION Chemistry experiment conductometric titration of hydrochloric Conductometric Titration Of Hcl And Naoh Before naoh is added, the conductance is high due to the presence of highly. Before naoh is added, the conductance is. In first, the solution of hcl is unknown molarity; 1) titration of strong acid (hcl) with strong base (naoh). Titration curve of a strong acid with a strong base, e.g. Some typical conductometric titration curves are: Strong acid with. Conductometric Titration Of Hcl And Naoh.
From www.youtube.com
Conductometric titration of HCl and acetic acid mixture against Conductometric Titration Of Hcl And Naoh Some typical conductometric titration curves are: Titration curve of a strong acid with a strong base, e.g. 1) titration of strong acid (hcl) with strong base (naoh). Strong acid with a strong base, e.g. In this experiment you are going to perform a conductometric titration of a mixture of a strong acid and weak acid with a strong base. It. Conductometric Titration Of Hcl And Naoh.
From www.studypool.com
SOLUTION Conductometric titration of hcl vs naoh Studypool Conductometric Titration Of Hcl And Naoh In first, the solution of hcl is unknown molarity; 1) titration of strong acid (hcl) with strong base (naoh). Before naoh is added, the conductance is high due to the presence of highly. Strong acid (hcl) and strong base (naoh) the observed shape of the titration curve (1) can be explained as follows: Titration curve of a strong acid with. Conductometric Titration Of Hcl And Naoh.
From www.toppr.com
Conductometric titration curve of equimolar mixture of a HCl and HCN Conductometric Titration Of Hcl And Naoh Before naoh is added, the conductance is high due to the presence of highly. Strong acid with a strong base, e.g. It is possible to find the concentration of. Some typical conductometric titration curves are: 1) titration of strong acid (hcl) with strong base (naoh). Titration curve of a strong acid with a strong base, e.g. In first, the solution. Conductometric Titration Of Hcl And Naoh.
From www.slideserve.com
PPT TITRATION CURVES PowerPoint Presentation ID1130069 Conductometric Titration Of Hcl And Naoh In first, the solution of hcl is unknown molarity; Before naoh is added, the conductance is high due to the presence of highly. 1) titration of strong acid (hcl) with strong base (naoh). Titration curve of a strong acid with a strong base, e.g. Strong acid (hcl) and strong base (naoh) the observed shape of the titration curve (1) can. Conductometric Titration Of Hcl And Naoh.
From www.numerade.com
SOLVEDConductometric titration curve of a equimolar mixture of a HCl Conductometric Titration Of Hcl And Naoh Strong acid (hcl) and strong base (naoh) the observed shape of the titration curve (1) can be explained as follows: Strong acid with a strong base, e.g. Titration curve of a strong acid with a strong base, e.g. In this experiment you are going to perform a conductometric titration of a mixture of a strong acid and weak acid with. Conductometric Titration Of Hcl And Naoh.
From www.researchgate.net
Representative example of conductometric titration from CNC batch 3 Conductometric Titration Of Hcl And Naoh Strong acid with a strong base, e.g. 1) titration of strong acid (hcl) with strong base (naoh). Some typical conductometric titration curves are: Strong acid (hcl) and strong base (naoh) the observed shape of the titration curve (1) can be explained as follows: It is possible to find the concentration of. Before naoh is added, the conductance is. In this. Conductometric Titration Of Hcl And Naoh.
From www.youtube.com
Conductometric titration of HCl solution with NaOH solution. YouTube Conductometric Titration Of Hcl And Naoh Titration curve of a strong acid with a strong base, e.g. Strong acid with a strong base, e.g. Before naoh is added, the conductance is. Some typical conductometric titration curves are: It is possible to find the concentration of. In first, the solution of hcl is unknown molarity; Strong acid (hcl) and strong base (naoh) the observed shape of the. Conductometric Titration Of Hcl And Naoh.
From www.youtube.com
Conductometric titration of HCl, Acetic Acid and copper sulphate Conductometric Titration Of Hcl And Naoh Strong acid (hcl) and strong base (naoh) the observed shape of the titration curve (1) can be explained as follows: In this experiment you are going to perform a conductometric titration of a mixture of a strong acid and weak acid with a strong base. It is possible to find the concentration of. Strong acid with a strong base, e.g.. Conductometric Titration Of Hcl And Naoh.
From klaimjxhd.blob.core.windows.net
Titration Between Hcl And Naoh at Tamika Spear blog Conductometric Titration Of Hcl And Naoh Strong acid with a strong base, e.g. Some typical conductometric titration curves are: Strong acid (hcl) and strong base (naoh) the observed shape of the titration curve (1) can be explained as follows: Titration curve of a strong acid with a strong base, e.g. In this experiment you are going to perform a conductometric titration of a mixture of a. Conductometric Titration Of Hcl And Naoh.
From mariela-kcarroll.blogspot.com
Titration Curve of Hcl and Naoh Conductometric Titration Of Hcl And Naoh Before naoh is added, the conductance is. In first, the solution of hcl is unknown molarity; 1) titration of strong acid (hcl) with strong base (naoh). In this experiment you are going to perform a conductometric titration of a mixture of a strong acid and weak acid with a strong base. Some typical conductometric titration curves are: Before naoh is. Conductometric Titration Of Hcl And Naoh.
From www.youtube.com
conductometric titration curve of a equimolar mixture of a HCl and HCN Conductometric Titration Of Hcl And Naoh Before naoh is added, the conductance is. In this experiment you are going to perform a conductometric titration of a mixture of a strong acid and weak acid with a strong base. 1) titration of strong acid (hcl) with strong base (naoh). Titration curve of a strong acid with a strong base, e.g. Strong acid (hcl) and strong base (naoh). Conductometric Titration Of Hcl And Naoh.
From chemistnotes.com
Conductometric titration, easy principle, curves, 3 advantages Conductometric Titration Of Hcl And Naoh Before naoh is added, the conductance is high due to the presence of highly. Strong acid with a strong base, e.g. In this experiment you are going to perform a conductometric titration of a mixture of a strong acid and weak acid with a strong base. Before naoh is added, the conductance is. 1) titration of strong acid (hcl) with. Conductometric Titration Of Hcl And Naoh.
From loehmowzt.blob.core.windows.net
Indicator Used In Titration Of Naoh And Hcl at Dan Carlson blog Conductometric Titration Of Hcl And Naoh Strong acid with a strong base, e.g. Titration curve of a strong acid with a strong base, e.g. Some typical conductometric titration curves are: It is possible to find the concentration of. In first, the solution of hcl is unknown molarity; In this experiment you are going to perform a conductometric titration of a mixture of a strong acid and. Conductometric Titration Of Hcl And Naoh.
From www.studypool.com
SOLUTION Section 8 conductometric titration of hcl vs naoh Studypool Conductometric Titration Of Hcl And Naoh In this experiment you are going to perform a conductometric titration of a mixture of a strong acid and weak acid with a strong base. 1) titration of strong acid (hcl) with strong base (naoh). It is possible to find the concentration of. Strong acid (hcl) and strong base (naoh) the observed shape of the titration curve (1) can be. Conductometric Titration Of Hcl And Naoh.
From www.embibe.com
Which of following plots will not be obtained for a conductometric Conductometric Titration Of Hcl And Naoh In first, the solution of hcl is unknown molarity; Titration curve of a strong acid with a strong base, e.g. Some typical conductometric titration curves are: Before naoh is added, the conductance is. It is possible to find the concentration of. Strong acid (hcl) and strong base (naoh) the observed shape of the titration curve (1) can be explained as. Conductometric Titration Of Hcl And Naoh.
From www.numerade.com
SOLVED The following data is from a conductometric titration of 10.00 Conductometric Titration Of Hcl And Naoh It is possible to find the concentration of. Some typical conductometric titration curves are: Before naoh is added, the conductance is high due to the presence of highly. Titration curve of a strong acid with a strong base, e.g. Before naoh is added, the conductance is. In this experiment you are going to perform a conductometric titration of a mixture. Conductometric Titration Of Hcl And Naoh.
From www.researchgate.net
Titration of HCl (0.1M) against NaOH (0.1M) Download Scientific Diagram Conductometric Titration Of Hcl And Naoh In first, the solution of hcl is unknown molarity; It is possible to find the concentration of. Strong acid (hcl) and strong base (naoh) the observed shape of the titration curve (1) can be explained as follows: Before naoh is added, the conductance is high due to the presence of highly. 1) titration of strong acid (hcl) with strong base. Conductometric Titration Of Hcl And Naoh.
From www.studypool.com
SOLUTION Section 8 conductometric titration of hcl vs naoh Studypool Conductometric Titration Of Hcl And Naoh In this experiment you are going to perform a conductometric titration of a mixture of a strong acid and weak acid with a strong base. Before naoh is added, the conductance is high due to the presence of highly. Strong acid (hcl) and strong base (naoh) the observed shape of the titration curve (1) can be explained as follows: Strong. Conductometric Titration Of Hcl And Naoh.
From www.studypool.com
SOLUTION Chemistry experiment conductometric titration of hydrochloric Conductometric Titration Of Hcl And Naoh Some typical conductometric titration curves are: In first, the solution of hcl is unknown molarity; Titration curve of a strong acid with a strong base, e.g. Before naoh is added, the conductance is. Strong acid with a strong base, e.g. It is possible to find the concentration of. 1) titration of strong acid (hcl) with strong base (naoh). In this. Conductometric Titration Of Hcl And Naoh.
From www.numerade.com
SOLVEDConductometric titration curve of an equimolar mixture of a HCl Conductometric Titration Of Hcl And Naoh In first, the solution of hcl is unknown molarity; 1) titration of strong acid (hcl) with strong base (naoh). Strong acid with a strong base, e.g. Some typical conductometric titration curves are: Before naoh is added, the conductance is high due to the presence of highly. Titration curve of a strong acid with a strong base, e.g. It is possible. Conductometric Titration Of Hcl And Naoh.
From www.studypool.com
SOLUTION Section 8 conductometric titration of hcl vs naoh Studypool Conductometric Titration Of Hcl And Naoh In this experiment you are going to perform a conductometric titration of a mixture of a strong acid and weak acid with a strong base. Before naoh is added, the conductance is high due to the presence of highly. Strong acid with a strong base, e.g. In first, the solution of hcl is unknown molarity; Titration curve of a strong. Conductometric Titration Of Hcl And Naoh.
From www.chegg.com
Solved Conductometric titration of HCl and ACOH with NaOH. Conductometric Titration Of Hcl And Naoh Some typical conductometric titration curves are: In this experiment you are going to perform a conductometric titration of a mixture of a strong acid and weak acid with a strong base. It is possible to find the concentration of. Strong acid (hcl) and strong base (naoh) the observed shape of the titration curve (1) can be explained as follows: In. Conductometric Titration Of Hcl And Naoh.
From klaimjxhd.blob.core.windows.net
Titration Between Hcl And Naoh at Tamika Spear blog Conductometric Titration Of Hcl And Naoh 1) titration of strong acid (hcl) with strong base (naoh). Some typical conductometric titration curves are: Strong acid (hcl) and strong base (naoh) the observed shape of the titration curve (1) can be explained as follows: Titration curve of a strong acid with a strong base, e.g. Before naoh is added, the conductance is. In this experiment you are going. Conductometric Titration Of Hcl And Naoh.
From www.youtube.com
Conductometric titration I strong acid (HCl) versus strong base Conductometric Titration Of Hcl And Naoh It is possible to find the concentration of. Before naoh is added, the conductance is high due to the presence of highly. Strong acid with a strong base, e.g. Strong acid (hcl) and strong base (naoh) the observed shape of the titration curve (1) can be explained as follows: In this experiment you are going to perform a conductometric titration. Conductometric Titration Of Hcl And Naoh.
From in.pinterest.com
Pin on ANALYTICAL CHEMISTRY Conductometric Titration Of Hcl And Naoh Some typical conductometric titration curves are: 1) titration of strong acid (hcl) with strong base (naoh). In first, the solution of hcl is unknown molarity; Before naoh is added, the conductance is high due to the presence of highly. In this experiment you are going to perform a conductometric titration of a mixture of a strong acid and weak acid. Conductometric Titration Of Hcl And Naoh.
From www.youtube.com
Conductometric titrations mixture of HCl and CH3COOH vs NaOH YouTube Conductometric Titration Of Hcl And Naoh Some typical conductometric titration curves are: Before naoh is added, the conductance is. Strong acid with a strong base, e.g. 1) titration of strong acid (hcl) with strong base (naoh). In first, the solution of hcl is unknown molarity; In this experiment you are going to perform a conductometric titration of a mixture of a strong acid and weak acid. Conductometric Titration Of Hcl And Naoh.
From www.researchgate.net
Conductometric titration of hydrochloric acid 0.1 M with sodium Conductometric Titration Of Hcl And Naoh Some typical conductometric titration curves are: Strong acid (hcl) and strong base (naoh) the observed shape of the titration curve (1) can be explained as follows: It is possible to find the concentration of. In first, the solution of hcl is unknown molarity; Before naoh is added, the conductance is. Strong acid with a strong base, e.g. 1) titration of. Conductometric Titration Of Hcl And Naoh.
From www.visionlearning.com
Ácidos y Bases I Chemistry Visionlearning Conductometric Titration Of Hcl And Naoh It is possible to find the concentration of. Strong acid with a strong base, e.g. Before naoh is added, the conductance is high due to the presence of highly. Some typical conductometric titration curves are: 1) titration of strong acid (hcl) with strong base (naoh). In first, the solution of hcl is unknown molarity; Before naoh is added, the conductance. Conductometric Titration Of Hcl And Naoh.
From www.numerade.com
SOLVED PART 2 CONDUCTOMETRIC TITRATION OF THE MIXTURE OF HCl AND Conductometric Titration Of Hcl And Naoh Strong acid (hcl) and strong base (naoh) the observed shape of the titration curve (1) can be explained as follows: Before naoh is added, the conductance is. In this experiment you are going to perform a conductometric titration of a mixture of a strong acid and weak acid with a strong base. Some typical conductometric titration curves are: 1) titration. Conductometric Titration Of Hcl And Naoh.
From www.doubtnut.com
The principle on conductometric titration is based in the fact that Conductometric Titration Of Hcl And Naoh Some typical conductometric titration curves are: In this experiment you are going to perform a conductometric titration of a mixture of a strong acid and weak acid with a strong base. Titration curve of a strong acid with a strong base, e.g. Before naoh is added, the conductance is. 1) titration of strong acid (hcl) with strong base (naoh). Before. Conductometric Titration Of Hcl And Naoh.
From www.studypool.com
SOLUTION Conductometric titration of hcl vs naoh Studypool Conductometric Titration Of Hcl And Naoh 1) titration of strong acid (hcl) with strong base (naoh). Titration curve of a strong acid with a strong base, e.g. Before naoh is added, the conductance is high due to the presence of highly. Strong acid with a strong base, e.g. Some typical conductometric titration curves are: Before naoh is added, the conductance is. In this experiment you are. Conductometric Titration Of Hcl And Naoh.
From www.researchgate.net
(PDF) Conductometric titration of hydrochloric acid by sodium hydroxide Conductometric Titration Of Hcl And Naoh Strong acid with a strong base, e.g. Before naoh is added, the conductance is. In this experiment you are going to perform a conductometric titration of a mixture of a strong acid and weak acid with a strong base. It is possible to find the concentration of. Strong acid (hcl) and strong base (naoh) the observed shape of the titration. Conductometric Titration Of Hcl And Naoh.
From www.studypool.com
SOLUTION Conductometric titration of hcl vs naoh Studypool Conductometric Titration Of Hcl And Naoh Before naoh is added, the conductance is high due to the presence of highly. Strong acid with a strong base, e.g. Some typical conductometric titration curves are: Before naoh is added, the conductance is. Strong acid (hcl) and strong base (naoh) the observed shape of the titration curve (1) can be explained as follows: In this experiment you are going. Conductometric Titration Of Hcl And Naoh.
From www.youtube.com
Conductometric Titration & Titration Curves // HSC Chemistry YouTube Conductometric Titration Of Hcl And Naoh In this experiment you are going to perform a conductometric titration of a mixture of a strong acid and weak acid with a strong base. Titration curve of a strong acid with a strong base, e.g. It is possible to find the concentration of. Strong acid (hcl) and strong base (naoh) the observed shape of the titration curve (1) can. Conductometric Titration Of Hcl And Naoh.