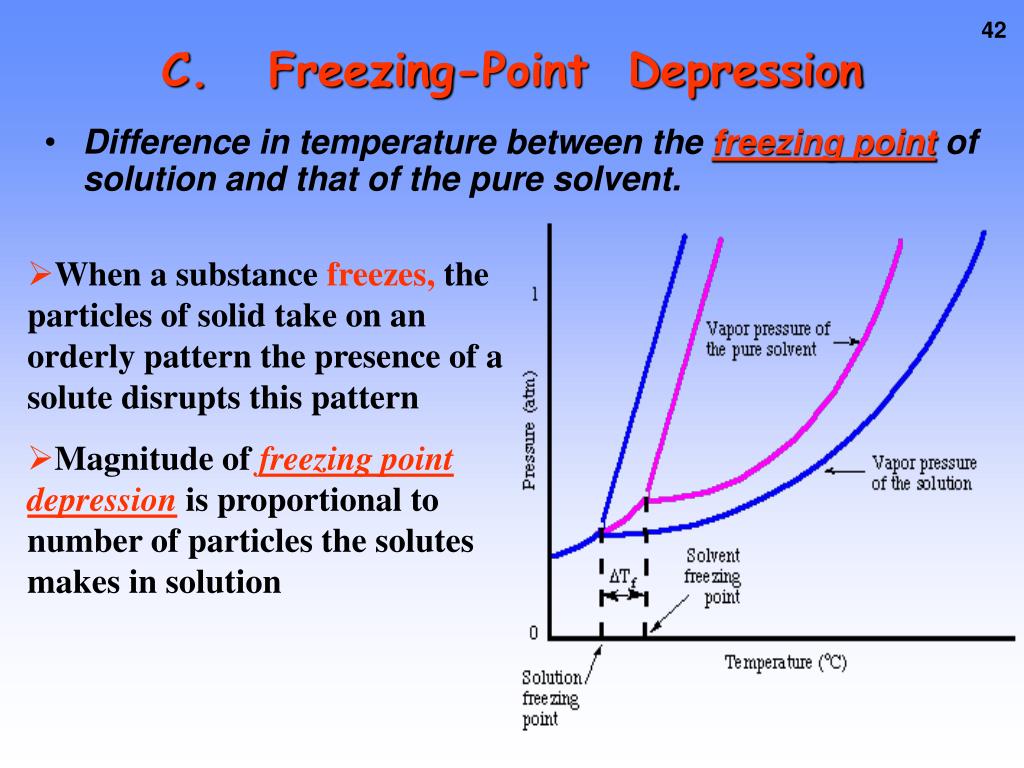
Does Increasing Pressure Lower Freezing Point . To maintain the solid and liquid phases in equilibrium, as pressure is increased above the triple point pressure, temperature must decrease slightly (to 273.15k if the pressure has increased to. Ice occupies more volume than liquid water, so squeezing. There are two ways that the higher pressure lowers the freezing temperature. The freezing point depression is the difference in the freezing points of the solution from the pure solvent. If you decrease the pressure, the freezing point of water will increase ever so slightly. To understand that the total number of nonvolatile solute particles determines the decrease in vapor pressure, increase in boiling point, and decrease in freezing point of a solution. This is true for any solute added. Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. As with the melting point, increased pressure usually raises the freezing. From 0° c at 1 atm pressure it will increase up to 0.01° c at. The freezing point is the temperature at which the liquid solvent and solid solvent are at equilibrium, so that their. Freezing point, temperature at which a liquid becomes a solid.
from www.slideserve.com
Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. To maintain the solid and liquid phases in equilibrium, as pressure is increased above the triple point pressure, temperature must decrease slightly (to 273.15k if the pressure has increased to. Ice occupies more volume than liquid water, so squeezing. The freezing point depression is the difference in the freezing points of the solution from the pure solvent. As with the melting point, increased pressure usually raises the freezing. To understand that the total number of nonvolatile solute particles determines the decrease in vapor pressure, increase in boiling point, and decrease in freezing point of a solution. The freezing point is the temperature at which the liquid solvent and solid solvent are at equilibrium, so that their. If you decrease the pressure, the freezing point of water will increase ever so slightly. Freezing point, temperature at which a liquid becomes a solid. From 0° c at 1 atm pressure it will increase up to 0.01° c at.
PPT Physical Properties of Solutions Review and Colligative
Does Increasing Pressure Lower Freezing Point To maintain the solid and liquid phases in equilibrium, as pressure is increased above the triple point pressure, temperature must decrease slightly (to 273.15k if the pressure has increased to. The freezing point is the temperature at which the liquid solvent and solid solvent are at equilibrium, so that their. To maintain the solid and liquid phases in equilibrium, as pressure is increased above the triple point pressure, temperature must decrease slightly (to 273.15k if the pressure has increased to. Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. From 0° c at 1 atm pressure it will increase up to 0.01° c at. Ice occupies more volume than liquid water, so squeezing. The freezing point depression is the difference in the freezing points of the solution from the pure solvent. Freezing point, temperature at which a liquid becomes a solid. As with the melting point, increased pressure usually raises the freezing. This is true for any solute added. To understand that the total number of nonvolatile solute particles determines the decrease in vapor pressure, increase in boiling point, and decrease in freezing point of a solution. If you decrease the pressure, the freezing point of water will increase ever so slightly. There are two ways that the higher pressure lowers the freezing temperature.
From sciencenotes.org
Freezing Point Depression Formula and Definition Does Increasing Pressure Lower Freezing Point This is true for any solute added. Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. The freezing point is the temperature at which the liquid solvent and solid solvent are at equilibrium, so that their. From 0° c at 1 atm pressure it will increase up to 0.01° c. Does Increasing Pressure Lower Freezing Point.
From www.slideserve.com
PPT I. The Vapor Pressure of Solutions PowerPoint Presentation, free Does Increasing Pressure Lower Freezing Point The freezing point depression is the difference in the freezing points of the solution from the pure solvent. To understand that the total number of nonvolatile solute particles determines the decrease in vapor pressure, increase in boiling point, and decrease in freezing point of a solution. Freezing point depression is the lowering of the equilibrium freezing or melting temperature by. Does Increasing Pressure Lower Freezing Point.
From www.youtube.com
Freezing Point And Vapour Pressure Detailed Facts YouTube Does Increasing Pressure Lower Freezing Point Freezing point, temperature at which a liquid becomes a solid. Ice occupies more volume than liquid water, so squeezing. This is true for any solute added. If you decrease the pressure, the freezing point of water will increase ever so slightly. As with the melting point, increased pressure usually raises the freezing. There are two ways that the higher pressure. Does Increasing Pressure Lower Freezing Point.
From www.jove.com
11369.jpg Does Increasing Pressure Lower Freezing Point To maintain the solid and liquid phases in equilibrium, as pressure is increased above the triple point pressure, temperature must decrease slightly (to 273.15k if the pressure has increased to. To understand that the total number of nonvolatile solute particles determines the decrease in vapor pressure, increase in boiling point, and decrease in freezing point of a solution. There are. Does Increasing Pressure Lower Freezing Point.
From www.slideserve.com
PPT Unit 4 Phases of Matter (Chapters 1314) PowerPoint Presentation Does Increasing Pressure Lower Freezing Point To maintain the solid and liquid phases in equilibrium, as pressure is increased above the triple point pressure, temperature must decrease slightly (to 273.15k if the pressure has increased to. Freezing point, temperature at which a liquid becomes a solid. The freezing point depression is the difference in the freezing points of the solution from the pure solvent. Ice occupies. Does Increasing Pressure Lower Freezing Point.
From chem.libretexts.org
13.8 FreezingPoint Depression and BoilingPoint Elevation of Does Increasing Pressure Lower Freezing Point From 0° c at 1 atm pressure it will increase up to 0.01° c at. The freezing point depression is the difference in the freezing points of the solution from the pure solvent. If you decrease the pressure, the freezing point of water will increase ever so slightly. Freezing point depression is the lowering of the equilibrium freezing or melting. Does Increasing Pressure Lower Freezing Point.
From www.researchgate.net
Variation curve of gas pressure at different freezing temperatures Does Increasing Pressure Lower Freezing Point The freezing point depression is the difference in the freezing points of the solution from the pure solvent. There are two ways that the higher pressure lowers the freezing temperature. To understand that the total number of nonvolatile solute particles determines the decrease in vapor pressure, increase in boiling point, and decrease in freezing point of a solution. To maintain. Does Increasing Pressure Lower Freezing Point.
From www.thoughtco.com
What Is the Freezing Point of Water? Does Increasing Pressure Lower Freezing Point Freezing point, temperature at which a liquid becomes a solid. To understand that the total number of nonvolatile solute particles determines the decrease in vapor pressure, increase in boiling point, and decrease in freezing point of a solution. If you decrease the pressure, the freezing point of water will increase ever so slightly. From 0° c at 1 atm pressure. Does Increasing Pressure Lower Freezing Point.
From www.slideserve.com
PPT Freezing Point Depression PowerPoint Presentation, free download Does Increasing Pressure Lower Freezing Point To understand that the total number of nonvolatile solute particles determines the decrease in vapor pressure, increase in boiling point, and decrease in freezing point of a solution. As with the melting point, increased pressure usually raises the freezing. This is true for any solute added. Ice occupies more volume than liquid water, so squeezing. To maintain the solid and. Does Increasing Pressure Lower Freezing Point.
From www.slideserve.com
PPT Chapter 16 “ Solutions ” PowerPoint Presentation, free download Does Increasing Pressure Lower Freezing Point There are two ways that the higher pressure lowers the freezing temperature. The freezing point depression is the difference in the freezing points of the solution from the pure solvent. This is true for any solute added. Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. The freezing point is. Does Increasing Pressure Lower Freezing Point.
From chemistnotes.com
Depression of Freezing Point Equation, Definition, and Applications Does Increasing Pressure Lower Freezing Point If you decrease the pressure, the freezing point of water will increase ever so slightly. To understand that the total number of nonvolatile solute particles determines the decrease in vapor pressure, increase in boiling point, and decrease in freezing point of a solution. Freezing point, temperature at which a liquid becomes a solid. The freezing point depression is the difference. Does Increasing Pressure Lower Freezing Point.
From chem.libretexts.org
13.8 FreezingPoint Depression and BoilingPoint Elevation of Does Increasing Pressure Lower Freezing Point There are two ways that the higher pressure lowers the freezing temperature. The freezing point depression is the difference in the freezing points of the solution from the pure solvent. This is true for any solute added. If you decrease the pressure, the freezing point of water will increase ever so slightly. From 0° c at 1 atm pressure it. Does Increasing Pressure Lower Freezing Point.
From www.slideserve.com
PPT Vapor Pressure of Solutions PowerPoint Presentation, free Does Increasing Pressure Lower Freezing Point The freezing point is the temperature at which the liquid solvent and solid solvent are at equilibrium, so that their. The freezing point depression is the difference in the freezing points of the solution from the pure solvent. Ice occupies more volume than liquid water, so squeezing. Freezing point, temperature at which a liquid becomes a solid. To maintain the. Does Increasing Pressure Lower Freezing Point.
From courses.lumenlearning.com
Colligative Properties Chemistry for Majors Does Increasing Pressure Lower Freezing Point This is true for any solute added. There are two ways that the higher pressure lowers the freezing temperature. Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. The freezing point is the temperature at which the liquid solvent and solid solvent are at equilibrium, so that their. From 0°. Does Increasing Pressure Lower Freezing Point.
From ksa.mytutorsource.com
Water Freezing Point Definition, Factors Affecting It & Supercooled Does Increasing Pressure Lower Freezing Point There are two ways that the higher pressure lowers the freezing temperature. Ice occupies more volume than liquid water, so squeezing. From 0° c at 1 atm pressure it will increase up to 0.01° c at. The freezing point is the temperature at which the liquid solvent and solid solvent are at equilibrium, so that their. This is true for. Does Increasing Pressure Lower Freezing Point.
From www.bartleby.com
Freezing Point of Water bartleby Does Increasing Pressure Lower Freezing Point There are two ways that the higher pressure lowers the freezing temperature. This is true for any solute added. Freezing point, temperature at which a liquid becomes a solid. The freezing point is the temperature at which the liquid solvent and solid solvent are at equilibrium, so that their. To maintain the solid and liquid phases in equilibrium, as pressure. Does Increasing Pressure Lower Freezing Point.
From inspectapedia.com
Mechanics & Forces of Freezing Water Effects of ice & freezing water on Does Increasing Pressure Lower Freezing Point Freezing point, temperature at which a liquid becomes a solid. If you decrease the pressure, the freezing point of water will increase ever so slightly. There are two ways that the higher pressure lowers the freezing temperature. This is true for any solute added. From 0° c at 1 atm pressure it will increase up to 0.01° c at. To. Does Increasing Pressure Lower Freezing Point.
From general.chemistrysteps.com
Freezing Point Depression Chemistry Steps Does Increasing Pressure Lower Freezing Point The freezing point is the temperature at which the liquid solvent and solid solvent are at equilibrium, so that their. There are two ways that the higher pressure lowers the freezing temperature. To understand that the total number of nonvolatile solute particles determines the decrease in vapor pressure, increase in boiling point, and decrease in freezing point of a solution.. Does Increasing Pressure Lower Freezing Point.
From www.ck12.org
Phase Diagrams CK12 Foundation Does Increasing Pressure Lower Freezing Point The freezing point depression is the difference in the freezing points of the solution from the pure solvent. The freezing point is the temperature at which the liquid solvent and solid solvent are at equilibrium, so that their. Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. There are two. Does Increasing Pressure Lower Freezing Point.
From preparatorychemistry.com
pH and Equilibrium Does Increasing Pressure Lower Freezing Point As with the melting point, increased pressure usually raises the freezing. From 0° c at 1 atm pressure it will increase up to 0.01° c at. Ice occupies more volume than liquid water, so squeezing. The freezing point is the temperature at which the liquid solvent and solid solvent are at equilibrium, so that their. To understand that the total. Does Increasing Pressure Lower Freezing Point.
From socratic.org
Calculate the change in freezing point for ice if the pressure changes Does Increasing Pressure Lower Freezing Point To maintain the solid and liquid phases in equilibrium, as pressure is increased above the triple point pressure, temperature must decrease slightly (to 273.15k if the pressure has increased to. The freezing point is the temperature at which the liquid solvent and solid solvent are at equilibrium, so that their. From 0° c at 1 atm pressure it will increase. Does Increasing Pressure Lower Freezing Point.
From wtamu.edu
Can water stay liquid below zero degrees Celsius? Science Questions Does Increasing Pressure Lower Freezing Point Ice occupies more volume than liquid water, so squeezing. From 0° c at 1 atm pressure it will increase up to 0.01° c at. The freezing point is the temperature at which the liquid solvent and solid solvent are at equilibrium, so that their. To understand that the total number of nonvolatile solute particles determines the decrease in vapor pressure,. Does Increasing Pressure Lower Freezing Point.
From www.leica-microsystems.com
Brief Introduction to HighPressure Freezing Science Lab Leica Does Increasing Pressure Lower Freezing Point From 0° c at 1 atm pressure it will increase up to 0.01° c at. If you decrease the pressure, the freezing point of water will increase ever so slightly. The freezing point depression is the difference in the freezing points of the solution from the pure solvent. This is true for any solute added. There are two ways that. Does Increasing Pressure Lower Freezing Point.
From www.expii.com
Phase Change Diagram of Water — Overview & Importance Expii Does Increasing Pressure Lower Freezing Point There are two ways that the higher pressure lowers the freezing temperature. If you decrease the pressure, the freezing point of water will increase ever so slightly. As with the melting point, increased pressure usually raises the freezing. Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. Ice occupies more. Does Increasing Pressure Lower Freezing Point.
From www.researchgate.net
Determination of freezing and melting points under pressure redrawn Does Increasing Pressure Lower Freezing Point The freezing point is the temperature at which the liquid solvent and solid solvent are at equilibrium, so that their. Ice occupies more volume than liquid water, so squeezing. There are two ways that the higher pressure lowers the freezing temperature. To understand that the total number of nonvolatile solute particles determines the decrease in vapor pressure, increase in boiling. Does Increasing Pressure Lower Freezing Point.
From sciencenotes.org
What Is the Freezing Point of Water? Fahrenheit, Celsius, and Kelvin Does Increasing Pressure Lower Freezing Point To maintain the solid and liquid phases in equilibrium, as pressure is increased above the triple point pressure, temperature must decrease slightly (to 273.15k if the pressure has increased to. Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. The freezing point is the temperature at which the liquid solvent. Does Increasing Pressure Lower Freezing Point.
From www.slideserve.com
PPT Physical Properties of Solutions Review and Colligative Does Increasing Pressure Lower Freezing Point To maintain the solid and liquid phases in equilibrium, as pressure is increased above the triple point pressure, temperature must decrease slightly (to 273.15k if the pressure has increased to. Ice occupies more volume than liquid water, so squeezing. The freezing point depression is the difference in the freezing points of the solution from the pure solvent. To understand that. Does Increasing Pressure Lower Freezing Point.
From mungfali.com
Freezing Point On Phase Diagram Does Increasing Pressure Lower Freezing Point Ice occupies more volume than liquid water, so squeezing. There are two ways that the higher pressure lowers the freezing temperature. To understand that the total number of nonvolatile solute particles determines the decrease in vapor pressure, increase in boiling point, and decrease in freezing point of a solution. As with the melting point, increased pressure usually raises the freezing.. Does Increasing Pressure Lower Freezing Point.
From ksa.mytutorsource.com
Water Freezing Point Definition, Factors Affecting It & Supercooled Does Increasing Pressure Lower Freezing Point There are two ways that the higher pressure lowers the freezing temperature. To understand that the total number of nonvolatile solute particles determines the decrease in vapor pressure, increase in boiling point, and decrease in freezing point of a solution. Ice occupies more volume than liquid water, so squeezing. Freezing point depression is the lowering of the equilibrium freezing or. Does Increasing Pressure Lower Freezing Point.
From www.slideserve.com
PPT Activity 2. Freezing Point of Liquids PowerPoint Presentation Does Increasing Pressure Lower Freezing Point This is true for any solute added. To maintain the solid and liquid phases in equilibrium, as pressure is increased above the triple point pressure, temperature must decrease slightly (to 273.15k if the pressure has increased to. As with the melting point, increased pressure usually raises the freezing. The freezing point depression is the difference in the freezing points of. Does Increasing Pressure Lower Freezing Point.
From owlcation.com
What Are the Freezing, Melting, and Boiling Points of Solids, Liquids Does Increasing Pressure Lower Freezing Point As with the melting point, increased pressure usually raises the freezing. There are two ways that the higher pressure lowers the freezing temperature. To understand that the total number of nonvolatile solute particles determines the decrease in vapor pressure, increase in boiling point, and decrease in freezing point of a solution. The freezing point depression is the difference in the. Does Increasing Pressure Lower Freezing Point.
From gabriela-classwork.blogspot.com
Science Class septiembre 2010 Does Increasing Pressure Lower Freezing Point To understand that the total number of nonvolatile solute particles determines the decrease in vapor pressure, increase in boiling point, and decrease in freezing point of a solution. Ice occupies more volume than liquid water, so squeezing. This is true for any solute added. To maintain the solid and liquid phases in equilibrium, as pressure is increased above the triple. Does Increasing Pressure Lower Freezing Point.
From www.differencebetween.com
Difference Between Freezing Point Depression and Boiling Point Does Increasing Pressure Lower Freezing Point If you decrease the pressure, the freezing point of water will increase ever so slightly. From 0° c at 1 atm pressure it will increase up to 0.01° c at. The freezing point depression is the difference in the freezing points of the solution from the pure solvent. As with the melting point, increased pressure usually raises the freezing. Freezing. Does Increasing Pressure Lower Freezing Point.
From shaunmwilliams.com
Chapter 11 Presentation Does Increasing Pressure Lower Freezing Point The freezing point depression is the difference in the freezing points of the solution from the pure solvent. From 0° c at 1 atm pressure it will increase up to 0.01° c at. As with the melting point, increased pressure usually raises the freezing. Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in. Does Increasing Pressure Lower Freezing Point.
From www.youtube.com
BoilingPoint Increase and FreezingPoint Decrease YouTube Does Increasing Pressure Lower Freezing Point To understand that the total number of nonvolatile solute particles determines the decrease in vapor pressure, increase in boiling point, and decrease in freezing point of a solution. As with the melting point, increased pressure usually raises the freezing. This is true for any solute added. Freezing point, temperature at which a liquid becomes a solid. The freezing point depression. Does Increasing Pressure Lower Freezing Point.