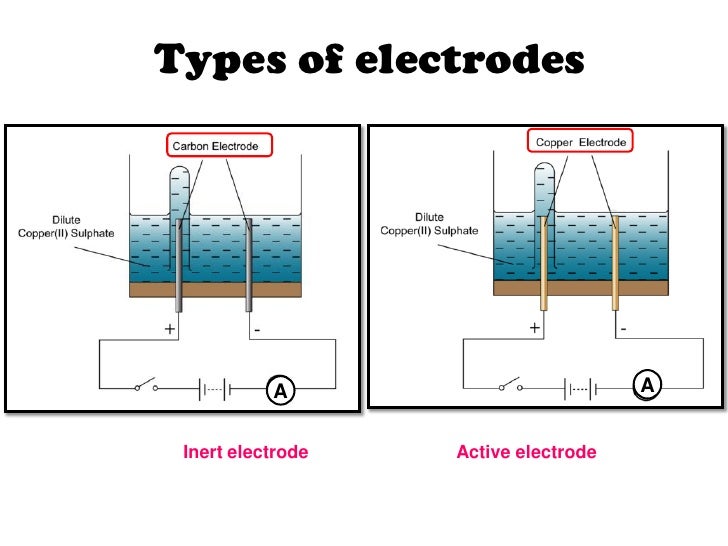
Inert Electrodes Examples . Electrochemical cells allow measurement and control of a redox reaction. Common examples of inert electrodes include materials like platinum, gold, and graphite. There are two types of electrodes, active. In order to hook up an external circuit you need have something to physically connect the wire to. If we place a variable resistance. Inert electrodes are often made from platinum or gold,. These metals possess characteristics that make them ideal for this purpose, such as. The reaction can be started and stopped by connecting or disconnecting the two electrodes. The electrodes that do not participate in the electrolysis reaction but help in transfer of electrons from cathode to. In this cell both zinc and copper electodes participate in the reactions and electrons are taken or given to this electrodes.
from www.slideshare.net
In order to hook up an external circuit you need have something to physically connect the wire to. Inert electrodes are often made from platinum or gold,. Common examples of inert electrodes include materials like platinum, gold, and graphite. The reaction can be started and stopped by connecting or disconnecting the two electrodes. There are two types of electrodes, active. If we place a variable resistance. These metals possess characteristics that make them ideal for this purpose, such as. In this cell both zinc and copper electodes participate in the reactions and electrons are taken or given to this electrodes. Electrochemical cells allow measurement and control of a redox reaction. The electrodes that do not participate in the electrolysis reaction but help in transfer of electrons from cathode to.
Electrolysis part 3 aqueous solution
Inert Electrodes Examples The reaction can be started and stopped by connecting or disconnecting the two electrodes. There are two types of electrodes, active. In this cell both zinc and copper electodes participate in the reactions and electrons are taken or given to this electrodes. In order to hook up an external circuit you need have something to physically connect the wire to. The reaction can be started and stopped by connecting or disconnecting the two electrodes. The electrodes that do not participate in the electrolysis reaction but help in transfer of electrons from cathode to. Common examples of inert electrodes include materials like platinum, gold, and graphite. Inert electrodes are often made from platinum or gold,. Electrochemical cells allow measurement and control of a redox reaction. If we place a variable resistance. These metals possess characteristics that make them ideal for this purpose, such as.
From www.slideserve.com
PPT ELECTROCHEMISTRY PowerPoint Presentation, free download ID6102398 Inert Electrodes Examples These metals possess characteristics that make them ideal for this purpose, such as. In this cell both zinc and copper electodes participate in the reactions and electrons are taken or given to this electrodes. The electrodes that do not participate in the electrolysis reaction but help in transfer of electrons from cathode to. There are two types of electrodes, active.. Inert Electrodes Examples.
From www.shalom-education.com
Electrolysis Using Inert Electrodes Edexcel GCSE Chemistry Revision Inert Electrodes Examples These metals possess characteristics that make them ideal for this purpose, such as. Inert electrodes are often made from platinum or gold,. Common examples of inert electrodes include materials like platinum, gold, and graphite. In this cell both zinc and copper electodes participate in the reactions and electrons are taken or given to this electrodes. Electrochemical cells allow measurement and. Inert Electrodes Examples.
From slidetodoc.com
Electroanalytical chemistry Potentiometry Voltammetry and Polarography Inert Electrodes Examples Inert electrodes are often made from platinum or gold,. If we place a variable resistance. In order to hook up an external circuit you need have something to physically connect the wire to. Common examples of inert electrodes include materials like platinum, gold, and graphite. In this cell both zinc and copper electodes participate in the reactions and electrons are. Inert Electrodes Examples.
From www.slideserve.com
PPT LECTURE 7 Electrochemistry. Types of electrodes and their using Inert Electrodes Examples There are two types of electrodes, active. The electrodes that do not participate in the electrolysis reaction but help in transfer of electrons from cathode to. Inert electrodes are often made from platinum or gold,. Electrochemical cells allow measurement and control of a redox reaction. In order to hook up an external circuit you need have something to physically connect. Inert Electrodes Examples.
From mungfali.com
Types Of Electrodes Inert Electrodes Examples In this cell both zinc and copper electodes participate in the reactions and electrons are taken or given to this electrodes. Common examples of inert electrodes include materials like platinum, gold, and graphite. The electrodes that do not participate in the electrolysis reaction but help in transfer of electrons from cathode to. Electrochemical cells allow measurement and control of a. Inert Electrodes Examples.
From www.slideserve.com
PPT electrolysis of solutions PowerPoint Presentation, free download Inert Electrodes Examples Common examples of inert electrodes include materials like platinum, gold, and graphite. These metals possess characteristics that make them ideal for this purpose, such as. There are two types of electrodes, active. Electrochemical cells allow measurement and control of a redox reaction. In this cell both zinc and copper electodes participate in the reactions and electrons are taken or given. Inert Electrodes Examples.
From studylibrenegado.z4.web.core.windows.net
Inert Electrodes Bbc Bitesize Inert Electrodes Examples There are two types of electrodes, active. In this cell both zinc and copper electodes participate in the reactions and electrons are taken or given to this electrodes. If we place a variable resistance. Common examples of inert electrodes include materials like platinum, gold, and graphite. Electrochemical cells allow measurement and control of a redox reaction. The reaction can be. Inert Electrodes Examples.
From www.youtube.com
4 Inert Electrodes YouTube Inert Electrodes Examples In order to hook up an external circuit you need have something to physically connect the wire to. If we place a variable resistance. The electrodes that do not participate in the electrolysis reaction but help in transfer of electrons from cathode to. Common examples of inert electrodes include materials like platinum, gold, and graphite. In this cell both zinc. Inert Electrodes Examples.
From chem.libretexts.org
11.2 Potentiometric Methods Chemistry LibreTexts Inert Electrodes Examples Inert electrodes are often made from platinum or gold,. The reaction can be started and stopped by connecting or disconnecting the two electrodes. These metals possess characteristics that make them ideal for this purpose, such as. In order to hook up an external circuit you need have something to physically connect the wire to. If we place a variable resistance.. Inert Electrodes Examples.
From www.youtube.com
Ch. 203 Inert Electrodes/Voltaic Cells YouTube Inert Electrodes Examples In order to hook up an external circuit you need have something to physically connect the wire to. Electrochemical cells allow measurement and control of a redox reaction. Inert electrodes are often made from platinum or gold,. In this cell both zinc and copper electodes participate in the reactions and electrons are taken or given to this electrodes. The electrodes. Inert Electrodes Examples.
From theclassroomsg.teachable.com
Lesson 1 Terminology, Inert electrodes, Molten Electrolyte Chem Inert Electrodes Examples In this cell both zinc and copper electodes participate in the reactions and electrons are taken or given to this electrodes. Inert electrodes are often made from platinum or gold,. There are two types of electrodes, active. Common examples of inert electrodes include materials like platinum, gold, and graphite. These metals possess characteristics that make them ideal for this purpose,. Inert Electrodes Examples.
From www.youtube.com
Chemistry Electrolysis (Inert & Active Electrodes) YouTube Inert Electrodes Examples In order to hook up an external circuit you need have something to physically connect the wire to. These metals possess characteristics that make them ideal for this purpose, such as. The reaction can be started and stopped by connecting or disconnecting the two electrodes. In this cell both zinc and copper electodes participate in the reactions and electrons are. Inert Electrodes Examples.
From studylibplimsoll.z21.web.core.windows.net
What Is An Inert Electrode Inert Electrodes Examples The electrodes that do not participate in the electrolysis reaction but help in transfer of electrons from cathode to. If we place a variable resistance. The reaction can be started and stopped by connecting or disconnecting the two electrodes. Electrochemical cells allow measurement and control of a redox reaction. Inert electrodes are often made from platinum or gold,. Common examples. Inert Electrodes Examples.
From www.slideserve.com
PPT LECTURE 7 Electrochemistry. Types of electrodes and their using Inert Electrodes Examples In order to hook up an external circuit you need have something to physically connect the wire to. Common examples of inert electrodes include materials like platinum, gold, and graphite. If we place a variable resistance. These metals possess characteristics that make them ideal for this purpose, such as. There are two types of electrodes, active. The electrodes that do. Inert Electrodes Examples.
From saylordotorg.github.io
Electrochemistry Inert Electrodes Examples Inert electrodes are often made from platinum or gold,. The reaction can be started and stopped by connecting or disconnecting the two electrodes. If we place a variable resistance. There are two types of electrodes, active. The electrodes that do not participate in the electrolysis reaction but help in transfer of electrons from cathode to. In this cell both zinc. Inert Electrodes Examples.
From www.differencebetween.com
Difference Between Active and Inert Electrodes Compare the Difference Inert Electrodes Examples Inert electrodes are often made from platinum or gold,. If we place a variable resistance. The electrodes that do not participate in the electrolysis reaction but help in transfer of electrons from cathode to. There are two types of electrodes, active. Common examples of inert electrodes include materials like platinum, gold, and graphite. Electrochemical cells allow measurement and control of. Inert Electrodes Examples.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Inert Electrodes Examples Electrochemical cells allow measurement and control of a redox reaction. Common examples of inert electrodes include materials like platinum, gold, and graphite. There are two types of electrodes, active. In order to hook up an external circuit you need have something to physically connect the wire to. Inert electrodes are often made from platinum or gold,. The electrodes that do. Inert Electrodes Examples.
From www.thoughtco.com
How to Define Anode and Cathode Inert Electrodes Examples In order to hook up an external circuit you need have something to physically connect the wire to. The reaction can be started and stopped by connecting or disconnecting the two electrodes. In this cell both zinc and copper electodes participate in the reactions and electrons are taken or given to this electrodes. If we place a variable resistance. The. Inert Electrodes Examples.
From www.slideserve.com
PPT potentiometry PowerPoint Presentation, free download ID7435364 Inert Electrodes Examples Inert electrodes are often made from platinum or gold,. The reaction can be started and stopped by connecting or disconnecting the two electrodes. Electrochemical cells allow measurement and control of a redox reaction. There are two types of electrodes, active. These metals possess characteristics that make them ideal for this purpose, such as. Common examples of inert electrodes include materials. Inert Electrodes Examples.
From www.youtube.com
Electrolysis inert electrode O level chemistry by Ms Chew YouTube Inert Electrodes Examples There are two types of electrodes, active. The electrodes that do not participate in the electrolysis reaction but help in transfer of electrons from cathode to. In order to hook up an external circuit you need have something to physically connect the wire to. Inert electrodes are often made from platinum or gold,. The reaction can be started and stopped. Inert Electrodes Examples.
From alevelchemistry.co.uk
Electrodes Facts, Summary & Definition Chemistry Revision Inert Electrodes Examples In this cell both zinc and copper electodes participate in the reactions and electrons are taken or given to this electrodes. There are two types of electrodes, active. These metals possess characteristics that make them ideal for this purpose, such as. In order to hook up an external circuit you need have something to physically connect the wire to. The. Inert Electrodes Examples.
From futuros.abrelatam.org
Explain Electrolysis Of Molten Copper Sulphate Using Inert, 47 OFF Inert Electrodes Examples If we place a variable resistance. These metals possess characteristics that make them ideal for this purpose, such as. In order to hook up an external circuit you need have something to physically connect the wire to. There are two types of electrodes, active. Inert electrodes are often made from platinum or gold,. The electrodes that do not participate in. Inert Electrodes Examples.
From quizunbestowed.z21.web.core.windows.net
Why Are Inert Electrodes Used In Electrolysis Inert Electrodes Examples Common examples of inert electrodes include materials like platinum, gold, and graphite. In this cell both zinc and copper electodes participate in the reactions and electrons are taken or given to this electrodes. These metals possess characteristics that make them ideal for this purpose, such as. There are two types of electrodes, active. In order to hook up an external. Inert Electrodes Examples.
From chem.libretexts.org
Standard Potentials Chemistry LibreTexts Inert Electrodes Examples If we place a variable resistance. The reaction can be started and stopped by connecting or disconnecting the two electrodes. There are two types of electrodes, active. These metals possess characteristics that make them ideal for this purpose, such as. The electrodes that do not participate in the electrolysis reaction but help in transfer of electrons from cathode to. In. Inert Electrodes Examples.
From icsechemistry16.blogspot.com
Electrolysis of Copper sulphate using inert electrodes Inert Electrodes Examples Electrochemical cells allow measurement and control of a redox reaction. The reaction can be started and stopped by connecting or disconnecting the two electrodes. In order to hook up an external circuit you need have something to physically connect the wire to. If we place a variable resistance. These metals possess characteristics that make them ideal for this purpose, such. Inert Electrodes Examples.
From www.youtube.com
voltaic cells example with inert electrodes YouTube Inert Electrodes Examples Common examples of inert electrodes include materials like platinum, gold, and graphite. In this cell both zinc and copper electodes participate in the reactions and electrons are taken or given to this electrodes. Inert electrodes are often made from platinum or gold,. If we place a variable resistance. Electrochemical cells allow measurement and control of a redox reaction. In order. Inert Electrodes Examples.
From www.slideshare.net
Electrolysis part 3 aqueous solution Inert Electrodes Examples There are two types of electrodes, active. Inert electrodes are often made from platinum or gold,. These metals possess characteristics that make them ideal for this purpose, such as. The reaction can be started and stopped by connecting or disconnecting the two electrodes. If we place a variable resistance. In order to hook up an external circuit you need have. Inert Electrodes Examples.
From www.slideserve.com
PPT REDOX EQM Half Cells with Inert Electrode PowerPoint Presentation Inert Electrodes Examples In this cell both zinc and copper electodes participate in the reactions and electrons are taken or given to this electrodes. There are two types of electrodes, active. The reaction can be started and stopped by connecting or disconnecting the two electrodes. If we place a variable resistance. In order to hook up an external circuit you need have something. Inert Electrodes Examples.
From exopopjam.blob.core.windows.net
Electrode Examples at Hillary Browning blog Inert Electrodes Examples The reaction can be started and stopped by connecting or disconnecting the two electrodes. If we place a variable resistance. Inert electrodes are often made from platinum or gold,. In order to hook up an external circuit you need have something to physically connect the wire to. Electrochemical cells allow measurement and control of a redox reaction. Common examples of. Inert Electrodes Examples.
From www.youtube.com
Inert electrodes Trew’s notes YouTube Inert Electrodes Examples Common examples of inert electrodes include materials like platinum, gold, and graphite. Inert electrodes are often made from platinum or gold,. The reaction can be started and stopped by connecting or disconnecting the two electrodes. The electrodes that do not participate in the electrolysis reaction but help in transfer of electrons from cathode to. In order to hook up an. Inert Electrodes Examples.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID6976551 Inert Electrodes Examples Electrochemical cells allow measurement and control of a redox reaction. Common examples of inert electrodes include materials like platinum, gold, and graphite. The electrodes that do not participate in the electrolysis reaction but help in transfer of electrons from cathode to. The reaction can be started and stopped by connecting or disconnecting the two electrodes. In this cell both zinc. Inert Electrodes Examples.
From www.youtube.com
Ch31 Redox Reactions in Chemical Cells (Inert electrode, Easy examples Inert Electrodes Examples The electrodes that do not participate in the electrolysis reaction but help in transfer of electrons from cathode to. In this cell both zinc and copper electodes participate in the reactions and electrons are taken or given to this electrodes. These metals possess characteristics that make them ideal for this purpose, such as. In order to hook up an external. Inert Electrodes Examples.
From studycopesettic.z21.web.core.windows.net
Inert Electrodes Gcse Inert Electrodes Examples Electrochemical cells allow measurement and control of a redox reaction. The electrodes that do not participate in the electrolysis reaction but help in transfer of electrons from cathode to. If we place a variable resistance. These metals possess characteristics that make them ideal for this purpose, such as. Common examples of inert electrodes include materials like platinum, gold, and graphite.. Inert Electrodes Examples.
From slidetodoc.com
Chemistry 30 Electrochemical Changes Electrolytic Cells Electrolytic Cells Inert Electrodes Examples Inert electrodes are often made from platinum or gold,. Common examples of inert electrodes include materials like platinum, gold, and graphite. These metals possess characteristics that make them ideal for this purpose, such as. There are two types of electrodes, active. In this cell both zinc and copper electodes participate in the reactions and electrons are taken or given to. Inert Electrodes Examples.
From www.slideserve.com
PPT Lets Set The Stage PowerPoint Presentation, free download ID Inert Electrodes Examples These metals possess characteristics that make them ideal for this purpose, such as. The reaction can be started and stopped by connecting or disconnecting the two electrodes. In order to hook up an external circuit you need have something to physically connect the wire to. If we place a variable resistance. In this cell both zinc and copper electodes participate. Inert Electrodes Examples.